-
Understanding primary immunodeficiency (PI)

Understanding PI
The more you understand about primary immunodeficiency (PI), the better you can manage it. Learn about PI diagnoses and treatment options.
-
Living with PI
-
Addressing mental health
-
Explaining your diagnosis
- General care
- Get support
- For parents and guardians
-
Managing workplace issues
- Navigating insurance
-
Traveling safely

Living with PI
Living with primary immunodeficiency (PI) can be challenging, but you’re not alone—many people with PI lead full and active lives. With the right support and resources, you can, too.
-
Addressing mental health
-
Get involved

Get involved
Be a hero for those with PI. Change lives by promoting primary immunodeficiency (PI) awareness and taking action in your community through advocacy, donating, volunteering, or fundraising.
-
Advancing research and clinical care
-
Research Grant Program
-
Consulting immunologist
-
Diagnosing PI
-
Getting prior authorization
-
Clinician education
-
Survey research
-
Participating in clinical trials

Advancing research and clinical care
Whether you’re a clinician, researcher, or an individual with primary immunodeficiency (PI), IDF has resources to help you advance the field. Get details on surveys, grants, and clinical trials.
-
Research Grant Program
In a landmark decision for the primary immunodeficiency (PI) community, the U.S. Food and Drug Administration (FDA) approved Waskyra (etuvetidigene autotemcel) to treat Wiskott-Aldrich syndrome (WAS). The decision makes WAS the first PI to have an approved gene therapy treatment in the U.S. Thirty-five years have passed since the world’s first gene therapy trial in 1990, treating two children with another life-threatening PI, adenosine deaminase deficiency severe combined immunodeficiency (ADA-SCID).
Waskyra is approved to treat patients aged six months and older with WAS who do not have a matched related donor for a bone marrow transplant (BMT). The FDA approval follows the European Medicines Agency’s (EMA) positive recommendation for the treatment in November, paving the way for access in the European Union as well.
“The FDA’s approval of Waskyra is a historic milestone for the Wiskott-Aldrich syndrome community. It reflects the power of global collaboration—families, clinicians, researchers, advocacy organizations, regulatory partners, and especially the patients and families whose courage made this possible,” said Dr. Sumathi Iyengar, co-founder and executive director of the Wiskott-Aldrich Foundation and Immune Deficiency Foundation (IDF) Community Advisory Board (CAB) member.
“The Wiskott-Aldrich Foundation has been involved since the earliest stages of these trials nearly 15 years ago, championing safer and less toxic therapies for the global WAS community. By partnering with clinicians, researchers, regulators, and families, the Foundation has played an active role in advancing gene therapy for patients with WAS.”
The FDA’s approval of the treatment is a remarkable achievement for the Italy-based nonprofit Fondazione Telethon, producer of Waskyra, because it marks the first time a nonprofit has led a gene therapy from laboratory development to regulatory approval.
“Gene therapy development—particularly for rare diseases—has historically faced significant scientific, regulatory, and economic hurdles that do not always align with traditional development models, driving the need for innovative, mission-driven development approaches such as the one exemplified by Telethon,” said Iyengar.
“We are proud to recognize Fondazione Telethon, the first nonprofit to bring a gene therapy to market, paving the way for other rare disorders. I am profoundly grateful to Telethon and to the team of clinicians and researchers at San Rafaelle Telethon Institute for Gene Therapy (SR-TIGET) led by Dr. Alessandro Aiuti, under whose leadership the gene therapy program started. Today marks a new era of hope for everyone living with WAS.”
WAS is a rare, life-threatening PI affecting mostly males, with an estimated incidence of 1 in 100,000 live births. It causes bleeding, eczema, recurrent infections, and increased susceptibility to autoimmune conditions and cancers. Genetic variants on the X chromosome in the WAS gene, named after the condition, cause WAS.
Before FDA approval of Waskyra, treatment options were limited to managing symptoms and BMT. BMT uses donor cells to provide the recipient with a functioning immune system. However, the donor must at least partially match the recipient for a number of genetic markers to make BMT feasible, and not all people with WAS can find a suitable donor. Instead, they live with the condition, treating infections and complications as they arise. Because gene therapy uses a patient’s own cells, instead of cells from a donor, these patients can now receive curative treatment.
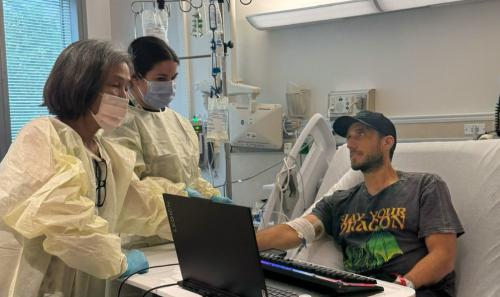
One of those who may benefit from the gene therapy treatment is Iyengar’s adult son. Unable to find a matched stem cell donor, her son takes preventative measures to manage complications from WAS. He is careful not to fall or injure himself, and when he gets an infection, he takes antibiotics. As a result of WAS, he has developed several autoimmune conditions as well.
“This gene therapy becomes even more important for patients like him who do not have a fully matched donor,” said Iyengar. “He's 27 years old and living with this devastating disease.”
Waskrya is a lentiviral gene therapy, which means it uses a hollowed-out virus to deliver a working copy of the WAS gene to patients’ cells. Doctors first take hematopoietic stem cells from a patient, then mix those cells with Waskyra. The lentiviral vector injects the working copy of WAS into the cells. Doctors then infuse the corrected cells back into the patient. The corrected cells move into the patient’s bone marrow and begin to grow and divide, providing the person with a healthy, functioning immune system.
“It cures the disease at a radical level, correcting it at the level of the gene where the problem starts,” explained Iyengar.
The hospital stay for gene therapy for WAS is about four to five weeks. Before receiving the corrected cells, the patient receives low-intensity chemotherapy, a lesser amount than what is given before BMT, to prepare their body and increase the chances that the gene therapy will work. Doctors infuse the corrected stem cells back into the patient after the chemotherapy. Engraftment, or successful growth of the cells, takes about 23 days on average.
“One of the biggest advantages of gene therapy is that the patient's own cells are used, and there is no risk of graft versus host disease, a condition where the donor cells attack the patient’s body. So, the post-transplant immunosuppressive therapy that is used for transplant is not needed for gene therapy, and that is a nice bonus to have,” said Iyengar.
Medical centers that will provide the treatment in the U.S. have not yet been finalized, nor have decisions been made related to the cost. With this resounding approval, hopes are high that public and private insurers will cover the therapy, said Iyengar.
“Access, affordability, is going to be a challenge, and we are already trying to face this head-on and see how we can work to make these therapies happen, and for the patients who need it,” said Iyengar.
The FDA assessed the safety and effectiveness of Waskyra based on multinational clinical studies that included 27 patients with severe WAS. The rate of severe infections decreased by 93% in the six to 18 months after treatment compared to the rate one year before treatment. Furthermore, the treatment reduced moderate and severe bleeding events by 60% in the first 12 months after treatment.
Iyengar said lentiviral gene therapy clinical trials for WAS started in 2010, and the research for those trials began a decade before. One of those early WAS researchers was Dr. Michael Blaese, who served on IDF’s Medical Advisory Committee (MAC) and then as its medical director.
A pioneer in gene therapy and a leading expert in WAS, Blaese passed away in November, just weeks before the FDA approved Waskyra. He would have been happy to see this treatment come to fruition, said Iyengar, who remembers cold-calling Blaese at the National Institutes of Health (NIH) shortly after her son’s diagnosis as an infant.
“Dr. Blaese was the one who started up the WAS program at the NIH. He followed one of the biggest cohorts of patients there with such caring, knowledge, and compassion. His knowledge of WAS was simply astounding, and he predicted that gene therapy would happen for WAS, and here we are today with gene therapy for WAS a reality,” said Iyengar.
“Dr. Blaese was a true giant who built the foundation and on whose shoulders we continue to grow and build. We are forever grateful to him.”
Iyengar said that over the last 15 years, as gene therapy research advanced, the community felt hopeful that it would someday be approved as a treatment, but also wondered, “Will it ever happen?” The WAS community took on the challenge, giving public testimony to regulators, and sharing their stories at the Externally Led-Patient Focused Drug Development Meeting (EL-PFDD) with the FDA to educate all the stakeholders.
“Going in we knew we had a very strong case, with excellent data based on successful results. Nevertheless, it is very emotional to see this finally happen for our families. We’ve been waiting decades for this therapy and we are thrilled to see it finally arrive,” said Iyengar.
2026 PI Conference
Join us June 25-27, 2026, in San Antonio, Texas, for a transformative experience where stories connect, voices unite, and journeys inspire. No matter where you are in your primary immunodeficiency journey, access expert-led education, meaningful connections, and cutting-edge insights from world-renowned immunologists.
Register nowRelated resources
Sign up for updates from IDF
Receive news and helpful resources to your cell phone or inbox. You can change or cancel your subscription at any time.




The Immune Deficiency Foundation improves the diagnosis, treatment, and quality of life for every person affected by primary immunodeficiency.
We foster a community that is connected, engaged, and empowered through advocacy, education, and research.