
Survey research
The IDF Survey Research Center provides timely data and analysis on issues important to the primary immunodeficiency (PI) community.

The IDF Survey Research Center provides timely data and analysis on issues important to the primary immunodeficiency (PI) community.
Since its founding in 2007, the IDF Survey Research Center has built a robust foundation of findings that are now considered authoritative resources for those in the primary immunodeficiency community. This critical quantitative data is collected from national surveys of patients, caregivers, and healthcare professionals. The research directly contributes to IDF's mission to improve the diagnosis, treatment, and quality of life of people affected by PI.
IDF survey data has been published in peer-reviewed journals and is often cited in academic papers, government-sponsored reports, and by the media. It has been used effectively to:
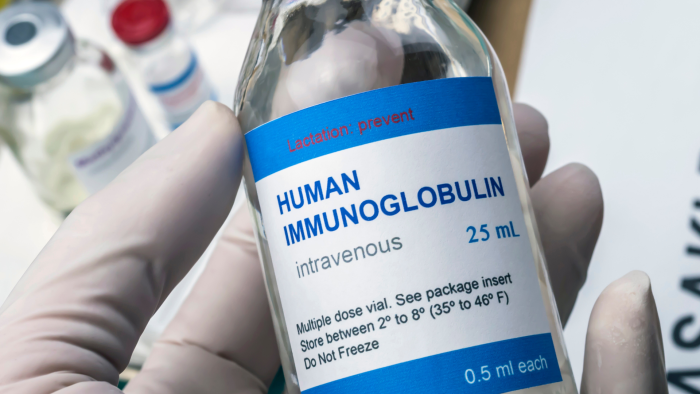
In 2024, the foundation conducted its fifth National Immunoglobulin (Ig) Treatment Survey to understand the experience of people with PI on Ig replacement therapy.
IDF maintains the complete anonymity of all of our survey respondents. All respondent-provided information is de-identified with all answers analyzed in the aggregate so as to prevent the identification of any one individual. Under no circumstances does IDF ever provide patient contact information to outside organizations without the patient’s prior consent.
IDF offers a research grant program to support patient-oriented PI research, funded by dollars raised from IDF Walk for PI teams and supporters.
Clinical trials help researchers develop safe, effective products that treat PI symptoms and other medical issues with limited side effects.
Receive news and helpful resources to your cell phone or inbox. You can change or cancel your subscription at any time.