
-
Understanding primary immunodeficiency (PI)

Understanding PI
The more you understand about primary immunodeficiency (PI), the better you can live with the disease or support others in your life with PI. Learn more about PI, including the various diagnoses and treatment options.
-
Living with PI
-
Addressing mental health
-
Explaining your diagnosis
- General care
- Get support
- For parents and guardians
-
Managing workplace issues
- Navigating insurance
-
Traveling safely

Living with PI
Living with primary immunodeficiency (PI) can be challenging, but you’re not alone—many people with PI lead full and active lives. With the right support and resources, you can, too.
-
Addressing mental health
-
Get involved

Get involved
Be a hero for those with PI. Change lives by promoting primary immunodeficiency (PI) awareness and taking action in your community through advocacy, donating, volunteering, or fundraising.
-
Advancing research and clinical care
-
Research Grant Program
-
Consulting immunologist
-
Diagnosing PI
-
Getting prior authorization
-
Clinician education
-
Survey research
-
Participating in clinical trials

Advancing research and clinical care
Whether you’re a clinician, researcher, or an individual with primary immunodeficiency (PI), IDF has resources to help you advance the field. Get details on surveys, grants, and clinical trials.
-
Research Grant Program
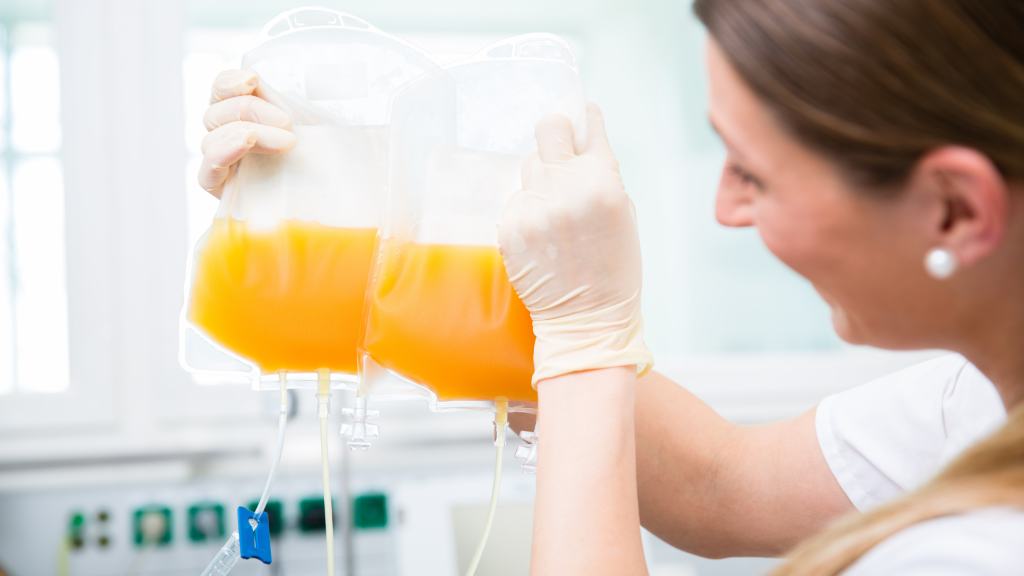
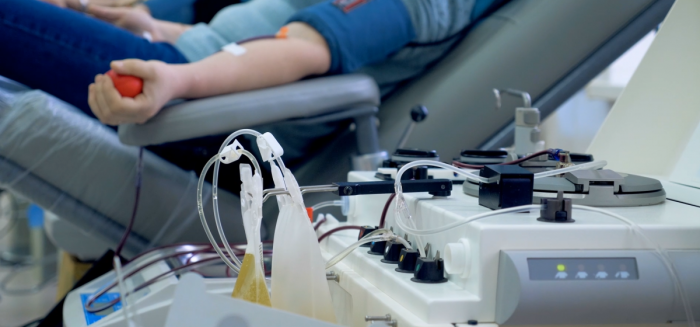
Many patients with primary immunodeficiency (PI) rely on plasma-based therapeutics (namely, immunoglobulin) that cannot be manufactured without the donation of source plasma—the liquid, cell-free component of blood—by volunteers. It takes approximately 130 plasma donations to produce one year’s supply of immunoglobulin for an adult with PI. Starting in late 2018 and continuing through the COVID-19 pandemic, there has been concern about potential immunoglobulin shortages due to increasing demand for plasma-based therapeutics and a decline in overall blood plasma donation.
IDF works to ensure adequate source plasma supply on two fronts.
- Address barriers that prevent plasma donation, such as state regulations that do not align with federal standards and border control measures that adversely affect the ability of non-U.S. nationals to donate plasma.
- Increase policymaker and public awareness of the need for plasma donation.
Why does plasma donation access and awareness matter?
A consistent supply of source plasma from donors is critical to maintaining the supply of plasma-derived therapies used by hundreds of thousands of individuals around the world. These therapies include immunoglobulin used by those with primary immunodeficiency and autoimmune disorders, clotting factors for those with hemophilia, and albumin used for burn and trauma victims.
Unfortunately, there are no synthetic substitutes for plasma-derived therapies. Only source plasma from volunteer donors can be used to create these life-saving medicines. While many people are aware of the need for blood donation, plasma donation has not received the same attention in the public sphere. Through its Plasma Hero campaign and the efforts of volunteer Plasma Ambassadors, IDF works to raise awareness among the general public about the need for and benefit of donating plasma.
What are the policy barriers to plasma donation?
With demand for source plasma steadily rising, removing unnecessary or unintentional barriers to plasma donation is critical.
Aligning state plasma donation regulations with federal standards
Regulation of plasma donation centers falls to states, and some states have enacted regulations that exceed the U.S. Food and Drug Administration’s (FDA) plasma donation regulations. For example, California requires licensed medical professionals to perform some of the necessary tests on potential plasma donors, while the FDA requires appropriately trained, but not necessarily licensed, professionals to perform these tests (note that Governor Newsom recently signed a bill to bring CA into alignment with federal standards). This federal standard allows plasma donation centers to operate efficiently with no impact on safety for either donors or recipients of donated plasma.
Such added regulatory burdens mean it is more difficult and costly to open plasma donation centers in these states, and as a result, some states, including New Hampshire, Vermont, Alaska, and Hawaii, have no plasma donation centers at all. Harmonizing state regulations with the federal standard would increase the pool of potential plasma donors by removing geographical barriers.
Use the interactive map below to explore the number of plasma centers in each state/territory per 100,000 residents. To see exact numbers, hover over (or tap and hold on mobile) a state/territory.
IDF's Director of State Policy, Jamie Sexton, provided testimony to the Connecticut legislature in support of harmonizing state regulations with federal standards in April 2022:
Overturning border control policy that limits plasma donations
At the federal level, a recent policy change enacted by U.S. Customs and Border Protection (CBP) has adversely affected cross-border plasma donation. In June 2021, CBP made the determination B1 or B2 visa holders could not be compensated for their time while donating plasma, classifying this activity as illegal “work for hire.” Previously, B1 and B2 visa holders were free to donate plasma and be compensated for their time, and it remains unclear why CBP reinterpreted this policy. As IDF estimates that up to 10% of the U.S. plasma supply comes from Mexican nationals who donate across the border with B1 or B2 visas, this barrier to donation could significantly limit source plasma supply.
IDF is working with a broad coalition of plasma donation centers, plasma therapy manufacturers, and patient organizations to overturn this policy. IDF has also reached out to facilitate dialogue between the U.S. Department of Health and Human Services (HHS) and the U.S. Department of Homeland Security (DHS) on the effects of the CBP policy. As of September 19, 2022, the United States District Court has granted a preliminary injunction that temporarily prevents CBP from enforcing its ban on B1 and B2 visa holders donating plasma.
Sign up for Action Alerts
When policymakers need to hear the PI community’s perspective, you will receive an Action Alert by email. Customize each alert with your information and hit send. It's that easy.

An initiative of IDF, Plasma Hero is a resource designed to guide individuals through the journey of plasma donation, encourage others to donate, and better support those who rely on plasma-based products.
Be a heroBecome a Plasma Ambassador
When you volunteer as a plasma ambassador, you help promote the importance of plasma donation by representing IDF and speaking about the organization’s mission and history to staff and donors at local plasma donation centers.
Sign up for updates from IDF
Receive news and helpful resources to your cell phone or inbox. You can change or cancel your subscription at any time.





The Immune Deficiency Foundation improves the diagnosis, treatment, and quality of life for every person affected by primary immunodeficiency.
We foster a community that is connected, engaged, and empowered through advocacy, education, and research.
Combined Charity Campaign | CFC# 66309



