
-
Understanding primary immunodeficiency (PI)

Understanding PI
The more you understand about primary immunodeficiency (PI), the better you can live with the disease or support others in your life with PI. Learn more about PI, including the various diagnoses and treatment options.
-
Living with PI
-
Addressing mental health
-
Explaining your diagnosis
- General care
- Get support
- For parents and guardians
-
Managing workplace issues
- Navigating insurance
-
Traveling safely

Living with PI
Living with primary immunodeficiency (PI) can be challenging, but you’re not alone—many people with PI lead full and active lives. With the right support and resources, you can, too.
-
Addressing mental health
-
Get involved

Get involved
Be a hero for those with PI. Change lives by promoting primary immunodeficiency (PI) awareness and taking action in your community through advocacy, donating, volunteering, or fundraising.
-
Advancing research and clinical care
-
Research Grant Program
-
Consulting immunologist
-
Diagnosing PI
-
Getting prior authorization
-
Clinician education
-
Survey research
-
Participating in clinical trials

Advancing research and clinical care
Whether you’re a clinician, researcher, or an individual with primary immunodeficiency (PI), IDF has resources to help you advance the field. Get details on surveys, grants, and clinical trials.
-
Research Grant Program
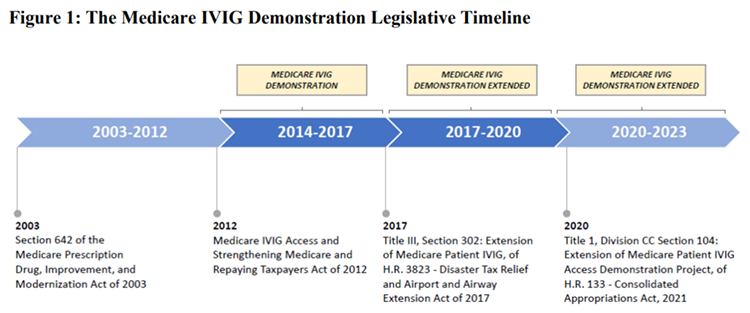
In 2012, Congress created a Medicare “demonstration” to pilot coverage of supplies and services, such as skilled nursing, needed for in-home intravenous immunoglobulin (IVIG) infusions. Since then, IDF has advocated for Congress to make such coverage a permanent Medicare benefit. A recently released Centers for Medicaid and Medicare Services (CMS) interim report bolsters the case for making the demonstration coverage permanent, documenting the health benefits to project enrollees.
Demonstration Project, https://innovation.cms.gov/data-and-reports/2022/ivig-updatedintrtc
Many Medicare patients on IVIG receive less than the clinically recommended number of infusions per year (13-18, based on treatment every 3-4 weeks) due to barriers associated with receiving treatment in outpatient facilities (e.g., doctor’s offices, hospitals) or the uncovered costs of receiving treatments at home. Demonstration enrollees self-reported improved access to IVIG therapy and, based on claims data, averaged 2.26 more treatments annually compared to non-enrollees. As a result, demonstration enrollees were 12.5% less likely than non-enrollees to receive infection-related care, indicating better management of their immune deficiency and better overall health. Indications of better overall health agree with the findings of other studies that have compared in-home versus outpatient IVIG infusion.
For many, moving onto Medicare from private insurance, which typically covers supplies and services for in-home IVIG treatment at least somewhat, has meant a surprise return to outpatient facilities for treatment. Said Cynthia Barber, a 73-year-old demonstration project enrollee in Illinois who has been on IVIG for 40 years, “When I went on Medicare, I was very upset they didn't cover the home infusions.”
Barber elaborated, “Receiving my treatments at home takes less time since the nurse is experienced. Also, I don't have a long drive and am not exposed to illnesses present in a hospital setting.”
“In this time of COVID-19, it gives me nightmares to think about going to another setting for my treatments, especially considering how risky it is for those with a PI,” said Gail Nelson, a project enrollee from Baton Rouge, LA.
Both Barber and Nelson echoed the advantages of in-home IVIG listed by both demonstration enrollees and healthcare providers in the report: decreased transportation barriers, reduced exposure to infection, increased treatment compliance, and improved infusion monitoring.
Nearly 4,000 Medicare beneficiaries with primary immunodeficiency have enrolled in the demonstration to date. The report clearly shows, while the demonstration impacts a relatively small population, it has had a positive impact on those beneficiaries’ health and well-being. The report estimates an additional cost of about $8,000 per enrollee per year for Medicare Part A and B services compared to non-enrollees. However, the report does not factor in the long-term cost savings associated with better overall enrollee health.
Long-time champions of the PI community, Representatives Kevin Brady (R-TX) and Doris Matsui (D-CA) are working on a provision to make the demonstration project coverage a permanent Medicare benefit. Representative Brady retires at the end of this Congress and there is an opportunity for the bill’s passage to augment his legacy. As Congress and the Congressional Budget Office (CBO) contemplate the costs and benefits of finally making in-home IVIG permanently accessible to Medicare beneficiaries, they should strongly consider both the benefit to the PI community and the cost savings resulting from improved health outcomes.
Related resources
Sign up for updates from IDF
Receive news and helpful resources to your cell phone or inbox. You can change or cancel your subscription at any time.





The Immune Deficiency Foundation improves the diagnosis, treatment, and quality of life for every person affected by primary immunodeficiency.
We foster a community that is connected, engaged, and empowered through advocacy, education, and research.
Combined Charity Campaign | CFC# 66309




