
-
Understanding primary immunodeficiency (PI)

Understanding PI
The more you understand about primary immunodeficiency (PI), the better you can live with the disease or support others in your life with PI. Learn more about PI, including the various diagnoses and treatment options.
-
Living with PI
-
Addressing mental health
-
Explaining your diagnosis
- General care
- Get support
- For parents and guardians
-
Managing workplace issues
- Navigating insurance
-
Traveling safely

Living with PI
Living with primary immunodeficiency (PI) can be challenging, but you’re not alone—many people with PI lead full and active lives. With the right support and resources, you can, too.
-
Addressing mental health
-
Get involved

Get involved
Be a hero for those with PI. Change lives by promoting primary immunodeficiency (PI) awareness and taking action in your community through advocacy, donating, volunteering, or fundraising.
-
Advancing research and clinical care
-
Research Grant Program
-
Consulting immunologist
-
Diagnosing PI
-
Getting prior authorization
-
Clinician education
-
Survey research
-
Participating in clinical trials

Advancing research and clinical care
Whether you’re a clinician, researcher, or an individual with primary immunodeficiency (PI), IDF has resources to help you advance the field. Get details on surveys, grants, and clinical trials.
-
Research Grant Program
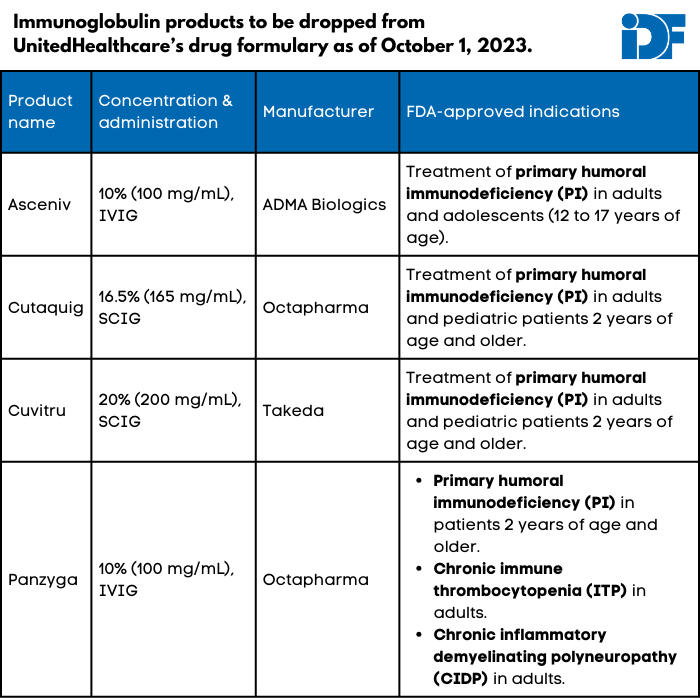
Update: In addition to most of its marketplace exchange plans, UnitedHealthcare (UHC) no longer covers Asceniv, Cuvitru, Cutaquig, and Panzyga, which are all immunoglobulin (Ig) products, in its commercial plans (i.e., those offered to individuals through their employers). UHC Medicare and Medicaid plans still cover all four Ig products.
The Immune Deficiency Foundation is working with UHC to reinstate coverage for these products. If you use Asceniv, Cuvitru, Cutaquig, or Panzyga because of a specific indication or because you cannot tolerate other Ig products, please share your story with us through Ask IDF.
Originally published on September 14, 2023
UnitedHealthcare (UHC), a private insurance company that provides health insurance to more than 25 million Americans nationwide, will be dropping four immunoglobulin (Ig) products on October 1, 2023: Asceniv, Cuvitru, Cutaquig, and Panzyga. These formulary changes will affect individual UnitedHealthcare plans (i.e., those obtained through a marketplace) except in Massachusetts, Nevada, and New York.
It is unclear if employer-based, Medicare, or Medicaid UHC plans are affected. UHC has not responded to questions seeking clarification.
None of the affected products has a biosimilar (generic version of a biologic) or interchangeable product recognized by the U.S. Food and Drug Administration. UHC's notice of the change declares, "Asceniv, Cutaquig, Cuvitru, and Panzyga are not medically necessary for the treatment of any diagnosis addressed within this policy," including primary immunodeficiency (PI), Guillain-Barré syndrome, chronic inflammatory demyelinating polyneuropathy (CIDP), and many others. UHC did not respond to questions about how these specific products were found to be not medically necessary despite their lack of biosimilars.
IDF and GBS|CIDP Foundation International, a global nonprofit organization supporting individuals and their families affected by GBS, CIDP, and related conditions, are jointly committed to serving the individuals affected by this policy. As we work with UHC and policymakers to reverse this decision, there are steps you can take if you will be impacted.
- Talk to your prescribing healthcare provider as soon as possible to choose a ‘backup’ Ig product should you lose access to one of the products listed above.
- Be prepared to file an appeal. If you have been prescribed one of the products above and have been on it stably for a while, you may be able to successfully appeal a coverage denial to UHC.
- Check out our step-by-step guide to filing an appeal.
- In particular, have your healthcare provider provide a short letter/documentation outlining why you are on your particular product, including any history of tolerability issues with similar products.
- Let us know if you are impacted. We rely on information and stories from you to put a face on decisions that negatively impact patients.
- If you use one of these products to treat a primary immunodeficiency, share your story through Ask IDF, and IDF will be in contact for additional details.
- If you use one of these products to treat GBS, CIDP, or MMN, contact the GBS|CIDP Foundation International.
Topics
Sign up for updates from IDF
Receive news and helpful resources to your cell phone or inbox. You can change or cancel your subscription at any time.





The Immune Deficiency Foundation improves the diagnosis, treatment, and quality of life for every person affected by primary immunodeficiency.
We foster a community that is connected, engaged, and empowered through advocacy, education, and research.
Combined Charity Campaign | CFC# 66309



