
-
Understanding primary immunodeficiency (PI)

Understanding PI
The more you understand about primary immunodeficiency (PI), the better you can live with the disease or support others in your life with PI. Learn more about PI, including the various diagnoses and treatment options.
-
Living with PI
-
Addressing mental health
-
Explaining your diagnosis
- General care
- Get support
- For parents and guardians
-
Managing workplace issues
- Navigating insurance
-
Traveling safely

Living with PI
Living with primary immunodeficiency (PI) can be challenging, but you’re not alone—many people with PI lead full and active lives. With the right support and resources, you can, too.
-
Addressing mental health
-
Get involved

Get involved
Be a hero for those with PI. Change lives by promoting primary immunodeficiency (PI) awareness and taking action in your community through advocacy, donating, volunteering, or fundraising.
-
Advancing research and clinical care
-
Research Grant Program
-
Consulting immunologist
-
Diagnosing PI
-
Getting prior authorization
-
Clinician education
-
Survey research
-
Participating in clinical trials

Advancing research and clinical care
Whether you’re a clinician, researcher, or an individual with primary immunodeficiency (PI), IDF has resources to help you advance the field. Get details on surveys, grants, and clinical trials.
-
Research Grant Program
Update
While babies born with either congenital athymia or severe combined immunodeficiency (SCID) both lack T cells, the causes of the two primary immunodeficiency disorders differ significantly, so it’s important to determine the correct diagnosis in order to move forward with the appropriate treatment.
This insight into congenital athymia is just one of many aspects of the disease described in a recent IDF Lunch & Learn, “Congenital Athymia 101,” presented by Dr. Elena Hsieh, University of Colorado School of Medicine Associate Professor of Pediatrics, Section of Allergy and Immunology and Department of Immunology and Microbiology, and Director for the Jeffrey Modell Center for Primary Immunodeficiencies.
In her presentation, Hsieh covered congenital athymia diagnosis, how congenital athymia is treated with thymus transplantation, genes affecting thymic development, and the social/emotional and medical impact of the condition on patients and families.
Congenital athymia is a condition in which a baby is born without a thymus. The thymus is a gland, located in front of the heart, responsible for teaching T cells how to support the immune system. Without T cells, a person’s immune system is severely compromised and a baby with congenital athymia will live only 2 to 3 years before succumbing to infection unless treated.
Diagnosis of congenital athymia is key to treatment and begins with newborn screening (NBS). If the baby's blood taken at NBS reveals the baby has low or no T cells, the baby is screened again, and if that screen is also abnormal, the baby is tested using flow cytometry to count the number of T cells in the circulation.
Both congenital athymia and SCID result in abnormal NBS and require further testing, called flow cytometry. Flow cytometry allows healthcare specialists to measure T cells, B cells, and NK cells. Babies with congenital athymia have missing T cells but make B and NK cells, whereas babies with SCID have missing T cells and may or may not make B and NK cells. Flow cytometry allows specialists to determine if missing T cells are due to congenital athymia or certain forms of SCID. However, some types of SCID having missing T cells only, and therefore can be indistinguishable from congenital athymia unless further genetic testing is pursued.
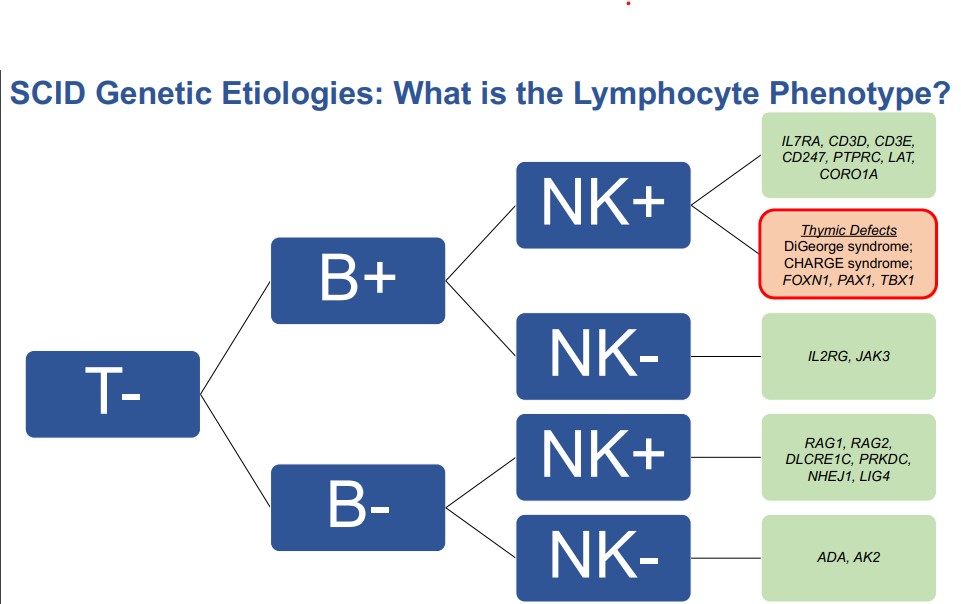
“If congenital athymia is a possibility, we have other features that can help us make the distinction. If there are additional syndromic comorbidities (health problems), that could very likely be associated with congenital athymia, but we still need verification, and we often perform genetic evaluations at this stage. We can either perform a chromosomal microarray or a genetic panel or both which, in most scenarios, we approach both at the same time,” explained Hsieh.
If flow cytometry and genetic analysis don’t lead to a definitive diagnosis, then researchers take the hematopoietic stem cells (cells from the bone marrow which develop into all types of blood cells including those which support the immune system, like T cells) from the baby and see how they progress in an artificial thymus system.
“If you put the cells in an artificial thymus and they successfully differentiate that means if had you a thymus then the cells could go on to become T cells, that means you actually have a missing thymus,” explained Hsieh. “However, if you put (the hematopoietic stem cells) in the artificial thymus and nothing happens, then that tells you that the problem is in the hematopoietic stem cell process and therefore that is SCID and not congenital athymia."
Making the distinction between SCID and congenital athymia is critical in how to proceed with treatment. SCID is treated with bone marrow transplant, enzyme replacement therapy, or clinical trial gene therapy. Congenital athymia is treated with thymus transplantation.
In thymus transplantation, specialists take donated thymus tissue and implant it in the thigh of the baby. Hematopoietic stem cells circulate through the thymus tissue and learn how to become healthy T cells.
“The reason why the thymus transplant works is because the thymus is the home of the education of T cells. T cells start being made in the bone marrow and they reach a stage at which they have to go and get educated, meaning that they go to the thymus, and they are told what is self and what is non-self. They become educated, and mature and then they leave the thymus,” explained Hsieh.
The pharmaceutical company Enzyvant manufactures the treatment, called Rethymic. Rethymic is produced by obtaining donor thymic tissue from healthy infants nine months or younger who have undergone heart surgery and had part of their thymus removed due to the surgery. Before implantation, the tissue is cultured for 13 to 20 days during which time the mature T cells are removed and the cells that will nurture the recipient’s new T cells are preserved. Importantly, an HLA tissue match between donor and recipient is not necessary for treatment.
Dr. Louise Markert and her research team at Duke University developed thymus transplantation (which led to the product, Rhythmic) in studies spanning over three decades. Rhythmic gained FDA approval in October 2021.
According to the Duke studies, immune reconstitution with thymic transplantation takes between six months to a year and the survival rate of children treated is about 80 percent.
Congenital athymia is a rare condition caused by variants in the genes that play a role in the development of the thymus while a baby is still in the embryonic stages.
“What genes would affect thymic development? We have essentially two broad categories. They are either genes that impact the development of the thymus specifically or there are genes that impact the development of the structure in the midline (an area from which the thymic develops). Genes that impact the development of the entire midline region are TBX1, CHD7, TBX2, and FOX13. Genes that affect thymic development are FOXN1 and PAX1,” said Hsieh.
In addition to a missing thymus and no healthy T cells, babies with congenital athymia may have other health problems depending on the affected gene.
FOXN1 deficiency results in a susceptibility to pneumonia, chronic diarrhea, failure to thrive, fungal infections, hair loss, and lack of B cells, which requires antibody replacement therapy. Babies may also have Omenn’s Syndrome, a condition in which the few T cells that do exist in the child attack the child’s own body, resulting in autologous graft versus host disease.
DiGeorge Syndrome and 22q11.2 deletion, caused by variants in a chunk of genetic material, not just one gene, can lead to an array of problems including poor antibody production, autoimmune complications, congenital anomalies, heart defects, parathyroid problems, calcium metabolism problems, facial dysmorphism, cleft palate, feeding difficulties, developmental delays, and psychiatric disorders.
CHARGE Syndrome due to CHD7 haploinsufficiency affects the development of the heart and ear and causes balance and coordination challenges, irregularly shaped pupils, and growth problems.
A 2021 survey of families of children with congenital athymia showed that the condition takes a heavy social and medical toll. Of those surveyed, 100 percent lived in isolation and reported experiencing an emotional burden, 78 percent had financial hardship, 72 percent were unable to meet friends and family, and 67 percent reported a lack of normalcy for siblings/family. The families also coped with significant medical procedures and hospitalizations including the following: radiology scans - 94 percent, sedation - 89 percent, feeding tube placement - 72 percent, central line placement - 67 percent, PICC line - 61 percent, and bronchoscopy - 56 percent.
Listen "Congenital Athymia 101."
___________________________________________________________________________________________
June 14, 2022
When it comes to immune system function, the thymus is a critical component. Located in front of the heart, the thymus is an organ that trains immature stem cells to become mature T cells when a baby is in a mother’s womb and continues to produce T cells until the child enters puberty.

Since T cells are essential to building an immune system and fighting infection, the thymus is crucial, particularly during the first two years of life when T cell production peaks.
While most babies have a thymus, every year about 17 to 24 babies in the U.S. are born without one, a condition called congenital athymia. Congenital athymia results in severe infection and other life-threatening health problems. Life expectancy is typically between 2 to 3 years old.
To further educate the community on this ultra-rare disease, the Immune Deficiency Foundation (IDF) will host Dr. Elena Hsieh from the Children’s Hospital Colorado for a discussion about congenital athymia and its associated genetic and syndromic disorders, including 22q11.2 (22q) deletion syndrome (which includes those with complete DiGeorge syndrome), CHARGE syndrome, and FOXN1 deficiency. The presentation takes place on Wednesday, June 22 at 1 p.m. Eastern Time and while the event is free, registration is required.
Causes of Congenital Athymia
Congenital athymia is most common in children who are born with 22q (which encompasses DiGeorge syndrome) and CHARGE. In CHARGE, congenital athymia is rare, but it is a more common feature in 22q.
In 22q, a child is missing a small piece of chromosome 22, which results in poor development of several body systems. In addition to poor immune function, a child with 22q may have growth delays, feeding problems, a heart defect, a cleft palate, calcium deficiencies, kidney problems, and developmental and learning challenges.
CHARGE also affects multiple body systems and is an acronym that stands for health issues related to the syndrome. Children with CHARGE have: Coloboma (a condition where a person is missing pieces of tissue in the structures that form the eye), Heart defects, Atresia of the choanal (narrowing of the back of the nasal cavity that causes difficulty breathing), Restriction of Growth and development, Ear abnormalities and deafness – and sometimes congenital athymia.
Other causes of congenital athymia include changes in certain genes, such as FOXN1, and diabetes in birth mothers.
Congenital athymia is identified through newborn screening by looking for T cell receptor incision circles or TRECS. A lack of TRECS means a lack of T cells, and those suspected to have congenital athymia are sent for further testing to determine if the lack of T cells is due to poor thymic function or a different condition, such as severe combined immunodeficiency (SCID).
Treatment for Congenital Athymia
Congenital athymia can be treated with antimicrobials and immunoglobulin therapy but those treatments only stave off infection and aren’t effective for long-term survival.
In October 2021, the Food and Drug Administration (FDA) approved a new treatment for congenital athymia called Rethymic (also known as allogeneic processed thymus tissue-agdc). Rethymic is donated thymus tissue that is processed and cultured, and then implanted into a child with congential athymia to replace the function of the missing thymus. Dr. Louise Markert and colleagues at Duke University spent the last 30 years developing the treatment in clinical trials.
When a patient’s stem cells travel to the implanted thymic tissue, the thymic tissue “teaches” the stem cells how to become T cells, a job the thymus normally performs. The thymic tissue is implanted in the patient’s thigh where good blood flow provides nutrients and oxygen.
In studies conducted by Markert and her colleagues, the thymic tissue transplant resulted in a 77 percent survival rate for children who would have otherwise succumbed to infection developed due to the lack of a thymus.
“Rethymic improved survival of children with congenital athymia, and most children treated with this product survived at least two years. Children treated with Rethymic who survive past the first year generally survive long-term,” according to the FDA press release. “Rethymic also reduced the frequency and severity of infections over time.”
While all patients are eligible for the treatment, a rigorous production process currently limits the number of children who can receive the treatment.
One consideration in the treatment production process is donor supply. The thymic tissue is only harvested from babies nine months or younger who have heart surgery requiring the removal of thymus tissue. (Babies at this age are less likely to have developed an infection, which could be passed on to the recipient.)
Another aspect that affects the treatment process is that the donor and patient must be close in size for the treatment to be successful. Each dosage of Rethymic is patient-specific.
Thirdly, the timing of the treatment must be exact and take place at Duke University Medical Center, the only location that performs the procedure. Once the thymic tissue is taken from the donor, it is delivered immediately to the manufacturing facility at Duke where it takes 14 to 21 days to process. The receiving child is flown in on short notice from a waiting list and the treatment must be administered to the child within three hours of the end of production.
Historically, during clinical trials, only about four or five patients were treated annually. However, medical and industry professionals expect the waiting list for Rethymic to decrease as production of the treatment increases.
Pharmaceutical company Enzyvant produces Rethymic and as with many pharmaceutical products derived from biological sources, Rhythmic is costly. In a statement, Enzyvant said that expenses for most patients are covered by government and commercial insurance providers. Enzyvant is currently in discussions to educate insurance coverage providers on the importance of the treatment and expedited coverage.
“Feedback has been positive, and we are excited about the possibilities for these children,” said the Enzyvant statement.
Patients wanting more information about coverage may enroll in Enzyvant Connect, a support program that provides the following:
Education and tools to guide caregivers through steps in the treatment journey
Assistance in working with insurers to help caregivers understand the coverage
Details on any out-of-pocket costs after insurance coverage and information on financial assistance programs
Sign up for updates from IDF
Receive news and helpful resources to your cell phone or inbox. You can change or cancel your subscription at any time.





The Immune Deficiency Foundation improves the diagnosis, treatment, and quality of life for every person affected by primary immunodeficiency.
We foster a community that is connected, engaged, and empowered through advocacy, education, and research.
Combined Charity Campaign | CFC# 66309



