-
Understanding primary immunodeficiency (PI)

Understanding PI
The more you understand about primary immunodeficiency (PI), the better you can manage it. Learn about PI diagnoses and treatment options.
-
Living with PI
-
Addressing mental health
-
Explaining your diagnosis
- General care
- Get support
- For parents and guardians
-
Managing workplace issues
- Navigating insurance
-
Traveling safely

Living with PI
Living with primary immunodeficiency (PI) can be challenging, but you’re not alone—many people with PI lead full and active lives. With the right support and resources, you can, too.
-
Addressing mental health
-
Get involved

Get involved
Be a hero for those with PI. Change lives by promoting primary immunodeficiency (PI) awareness and taking action in your community through advocacy, donating, volunteering, or fundraising.
-
Advancing research and clinical care
-
Research Grant Program
-
Consulting immunologist
-
Diagnosing PI
-
Getting prior authorization
-
Clinician education
-
Survey research
-
Participating in clinical trials

Advancing research and clinical care
Whether you’re a clinician, researcher, or an individual with primary immunodeficiency (PI), IDF has resources to help you advance the field. Get details on surveys, grants, and clinical trials.
-
Research Grant Program
Update: As of January 26, 2023, Evusheld is no longer authorized by the U.S. Food and Drug Administration (FDA) and will be unavailable to patients until further notice. This decision stems from data showing that Evusheld does not effectively neutralize many newer COVID-19 variants. FDA has revised Evusheld's emergency use authorization (EUA) to state that it is now only authorized for use if more than 10% of circulating variants are expected to be neutralized by it. The U.S. Centers for Disease Control and Prevention (CDC) currently predicts that variants that are not neutralized by Evusheld make up more than 90% of variants circulating in all areas of the U.S.
In light of the FDA's decision, the Centers for Disease Control and Prevention (CDC) reiterated its recommendations for those who are immunocompromised. The recommended prevention measures include masking, getting the bivalent COVID-19 booster vaccination, and knowing where and how to get tested and get antiviral medication in case of exposure.

https://www.cdc.gov/mmwr/volumes/72/wr/mm7205e3.htm?s_cid=mm7205e3_w.
January 10, 2022 Update
On January 6, 2023, the Food and Drug Administration (FDA) again updated information on the effectiveness of Evusheld, addressing COVID-19 subvariant XBB.1.5. The agency noted that although laboratory data is still in process, the similarity of XBB.1.5 to XBB in the areas of the virus that are targeted by Evusheld’s antibody mix makes it unlikely that Evusheld is effective against XBB.1.5. As of December 17, 2022, only about 24% of COVID-19 infections nationwide were from variants that have been shown to be neutralized by Evusheld like BA.5 and BN.1.
Responding to the alarm of immunocompromised individuals who relied on Evusheld’s protection, AstraZeneca announced clinical trials of a second-generation antibody mix for COVID-19 prevention. The new formulation retains one of the two original Evusheld antibodies, cilgavimab, and adds a second long-acting monoclonal antibody that is broadly neutralizing—that is, it neutralizes many different COVID-19 variants, including the subvariants of Omicron that Evusheld does not neutralize. The company hopes to make its new protective product available in the second half of 2023.
November 14, 2022 Update
The National Institutes of Health (NIH) COVID-19 Treatment Guidelines Panel released a statement advising that AstraZeneca’s Evusheld is likely ineffective against COVID-19 variants BA.2.75.2, BA.5.2.6, BF.7, BQ.1, and BQ.1.1 based on pseudovirus neutralization data from an unpublished preprint paper. This statement comes a little over a month after the FDA issued a similar warning that Evusheld likely does not neutralize the BA.4.6 variant (see Oct. 5, 2022 update).
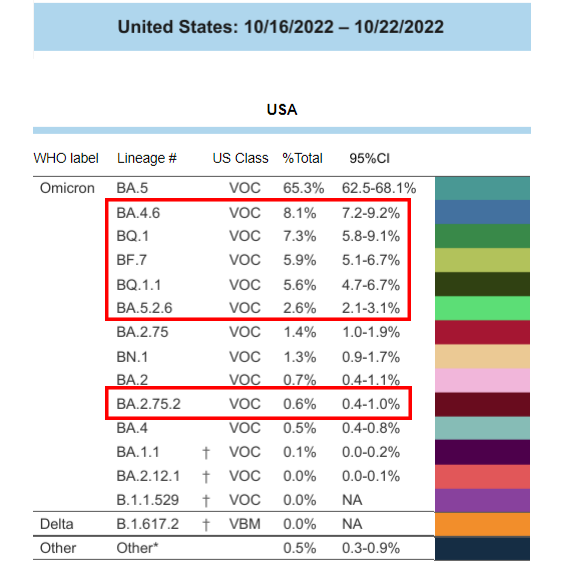
as of Oct. 22, 2022.Those that are likely not
neutralized by Evusheld are outlined in red.
As of October 22, 2022, the latest data available, variants that are unlikely to be neutralized by Evusheld were collectively responsible for 30% of COVID-19 infections across the U.S. A Centers for Disease Control and Prevention (CDC) model predicts those variants represent up to 61% of U.S. COVID-19 infections as of last Friday, November 12, 2022.
Both the NIH panel and the FDA still recommend Evusheld for people who are immunocompromised, but caution healthcare providers and patients to be aware that Evusheld’s protection against COVID-19 may not be as strong as it has been previously. Both also highlight the importance of other precautions, including receiving the bivalent COVID-19 vaccine booster (unless contraindicated), masking, and social distancing.
October 5, 2022 Update
The U.S. Food and Drug Administration (FDA) has issued a warning to patients and healthcare providers that there may be an increased risk of developing COVID-19 in areas where the Omicron subvariant BA.4.6 is circulating even after receiving Evusheld. The warning was issued in response to studies demonstrating that the two monoclonal antibodies in AstraZeneca’s Evusheld do not neutralize BA.4.6 .
As of October 1, 2022, the U.S. Centers for Disease Control and Prevention (CDC) reports that approximately 12.8% of sequenced COVID-19 infections are BA.4.6 in the U.S . Region 7, which covers Iowa, Kansas, Missouri, and Nebraska, currently has the highest proportion of BA.4.6 infections in the U.S. at 21.9%.
FDA posted the following updated documents addressing BA.4.6:
- Evusheld Fact Sheet for Healthcare Providers (updated 10/3/2022)
- Frequently Asked Questions on the EUA for Evusheld (updated 10/3/2022)
September 7, 2022 Update
The U.S. Centers for Disease Control and Prevention (CDC) has issued guidance for the timing of COVID-19 vaccination relative to the administration of Evusheld in patients who are immunocompromised. Patients should wait two weeks after their most recent vaccine dose before receiving Evusheld. However, there is no need to wait after receiving Evusheld to get your next recommended vaccine dose.
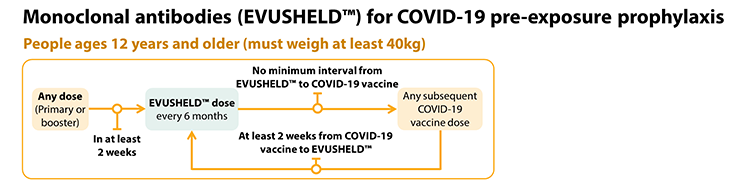
https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html#immunocompromised
July 15, 2022 Update
In late June 2022, the U.S. Food and Drug Administration (FDA) made two critical updates to their authorization and recommendations regarding Evusheld. First, the FDA authorized an extension on the shelf-life of refrigerated lots of Evusheld from 18 to 24 months based on data from the manufacturer, AstraZeneca. Given that many lots of the COVID-19 preventative were close to their original expiration dates, this extension ensures additional time for healthcare providers to get Evusheld to those who are immunocompromised.
Second, the FDA released a recommendation for re-dosing with Evusheld. Healthcare providers should prescribe another 300 mg dose (300 mg of each of the two monoclonal antibodies that make up Evusheld) every six months for as long as the patient in question needs COVID-19 protection.
February 25, 2022 Update
Data show that Evusheld retains neutralizing activity against Omicron variants BA.1 and BA.1.1 at a level comparable to natural infection, but lower than its neutralizing activity against previous COVID-19 variants. Accordingly, the U.S. Food and Drug Administration has updated its emergency use authorization (EUA) for Evusheld to recommend that the dose be doubled from its current level of 150 mg of each antibody (tixagevimab and cilgavimab) to 300 mg of each antibody. The antibodies will still be given as consecutive intramuscular shots during one healthcare visit. FDA is also recommending that patients who received Evusheld at the lower 150 mg dose secure a second 150 mg dose as soon as possible.
On February 14, 2022, prior to this dosing update, the U.S. government announced it had purchased an additional 500,000 doses, bringing the total available doses in the U.S. to 1.7 million. However, with the increase in recommended dosage, the number of doses is effectively cut in half to 850,000. Only the U.S. government can purchase Evusheld because of limitations in its EUA, which means that it cannot be made commercially available to pharmacies or doctors.
If your doctor has recommended that you receive Evusheld, but you have not been able to secure a dose (or cannot find a needed second dose), please contact us through AskIDF so that we can collect information and advocate for the government purchase and distribution of additional doses.
January 14, 2022 Update
The U.S. federal government has purchased an additional 500,000 doses of Evusheld in response to concerns that the original order of 700,000 doses would not be enough to cover the immunocompromised population in the U.S. The federal government is now shipping doses weekly rather than biweekly, as originally planned.
The National Institutes of Health (NIH) released treatment guidelines for Evusheld that specifically state that it is "not a substitute for COVID-19 vaccination and should not be used in unvaccinated individuals for whom COVID-19 vaccination is recommended." The guidelines also specifies that people with moderate to severe primary immunodeficiencies qualify to receive Evusheld under its emergency user authorization (EUA).
Finally, two studies that have not yet been peer reviewed, one from Oxford University and the other from Washington University, provide additional evidence that Evusheld retains neutralizing activity against the Omicron variant of SARS-CoV-2.
Originally published December 9, 2021
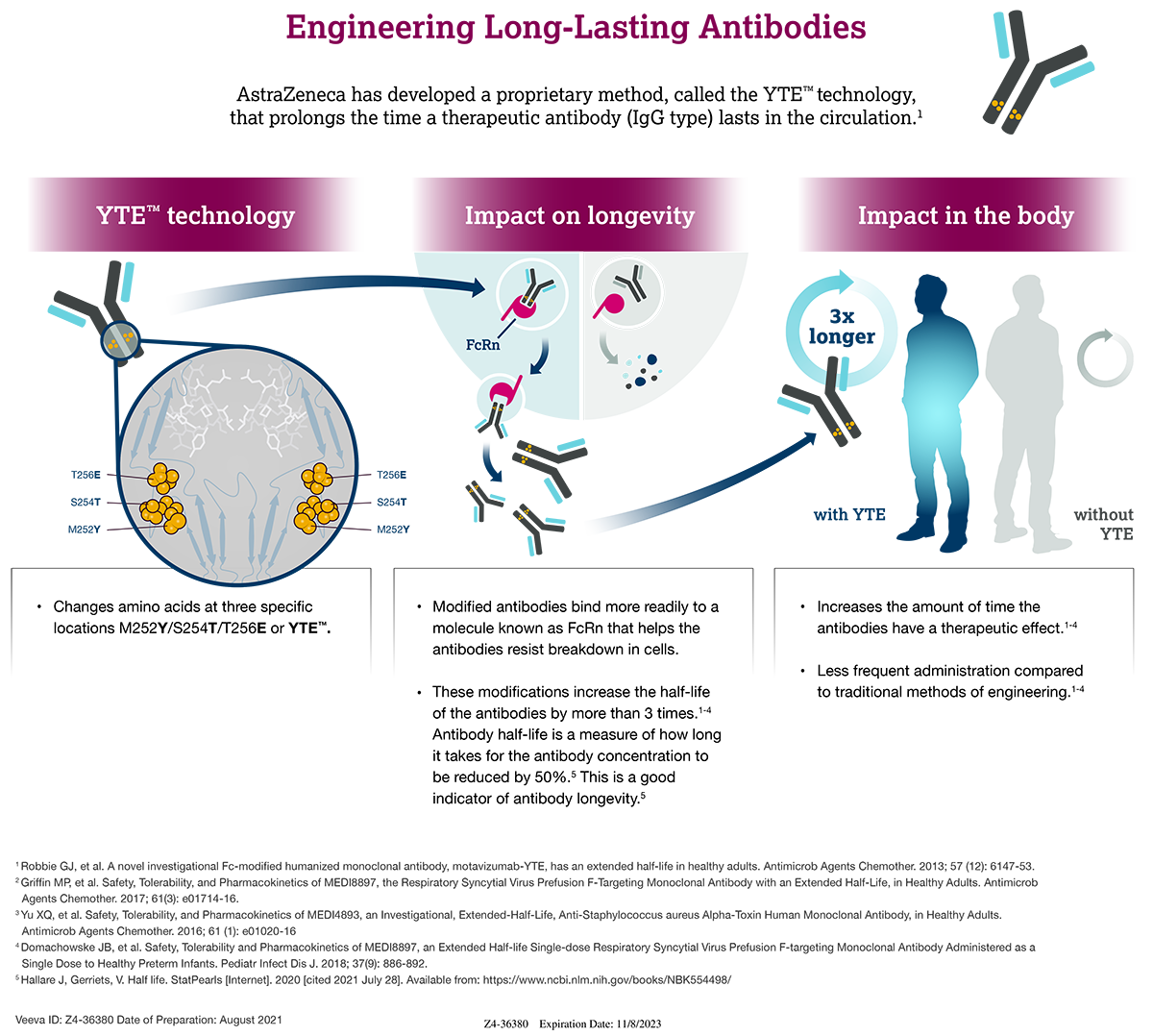
Evusheld, a combination of two long-acting monoclonal antibodies developed by AstraZeneca, has received emergency use authorization (EUA) as a prophylactic against COVID-19 from the U.S. Food and Drug Administration (FDA). It is the first monoclonal antibody product authorized specifically for use before any known exposure to the virus and could provide protection for up to six months.
“This new monoclonal antibody cocktail COVID-19 product is a game-changer for patients with PI who can not mount an adequate antibody response to the COVID vaccine,” said Dr. Mark Ballow, IDF’s Consulting Medical Director.
The EUA indicates that the drug is intended for those 12 and over who are moderately to severely immunocompromised, including those with moderate to severe primary immunodeficiency. It is not intended as a substitute for vaccination for the general public.
Initial availability will be through the federal government, which has purchased 700,000 doses to be distributed to states based on their populations. States will then determine distribution sites and will rely on forthcoming guidelines from the National Institutes of Health (NIH) on who is eligible to receive the drug. IDF is working closely with AstraZeneca and the government to ensure that those with PI will have access to Evusheld as quickly as possible.
In AstraZeneca’s PROVENT phase III trial, one dose of Evusheld provided 83% efficacy against symptomatic COVID-19 for six months in a population where 75% of trial participants had one or more risk factors for severe disease. The two antibodies, tixagevimab and cilgavimab, were each derived from convalescent sera from individuals recovered from COVID-19 infection. They are delivered as two sequential intramuscular injections.
The Omicron COVID-19 variant was not circulating when the PROVENT trial was conducted, but preliminary data from FDA scientists indicate that Evusheld may remain effective against the variant. AstraZeneca and others are completing additional studies to confirm the drug's effectiveness against Omicron.
The EUA is great news for our community, but patience is needed as we help guide this therapy through mandated processes. In the meantime, please maintain appropriate pandemic precautions. If you have not received a COVID-19 vaccine, please have a conversation with your physician to see if the vaccine is appropriate for you.
Read the latest updates on COVID-19 and PI.
Related resources
Sign up for updates from IDF
Receive news and helpful resources to your cell phone or inbox. You can change or cancel your subscription at any time.




The Immune Deficiency Foundation improves the diagnosis, treatment, and quality of life for every person affected by primary immunodeficiency.
We foster a community that is connected, engaged, and empowered through advocacy, education, and research.