
-
Understanding primary immunodeficiency (PI)

Understanding PI
The more you understand about primary immunodeficiency (PI), the better you can live with the disease or support others in your life with PI. Learn more about PI, including the various diagnoses and treatment options.
-
Living with PI
-
Addressing mental health
-
Explaining your diagnosis
- General care
- Get support
- For parents and guardians
-
Managing workplace issues
- Navigating insurance
-
Traveling safely

Living with PI
Living with primary immunodeficiency (PI) can be challenging, but you’re not alone—many people with PI lead full and active lives. With the right support and resources, you can, too.
-
Addressing mental health
-
Get involved

Get involved
Be a hero for those with PI. Change lives by promoting primary immunodeficiency (PI) awareness and taking action in your community through advocacy, donating, volunteering, or fundraising.
-
Advancing research and clinical care
-
Grants
-
IDF surveys
-
Participating in clinical trials
-
Diagnosing PI
-
Consulting immunologist
-
Clinician education

Advancing research and clinical care
Whether you’re a clinician, researcher, or an individual with primary immunodeficiency (PI), IDF has resources to help you advance the field. Get details on surveys, grants, and clinical trials.
-
Grants
Doctors pour drugs of which they know little, to cure diseases of which they know less, into patients of whom they know nothing.
- -Moliere, French actor & comic dramatist (1622-1673)
Although the above quote in 2021 may not be entirely accurate, it still holds true today. Tomorrow, with your help, we can change that.
When I first started at IDF in 2007, we were a small organization, with about 12 full-time employees and a multitude of volunteers. Research at the time for us consisted of the United States Immunodeficiency Network (USIDNET) and occasional surveys of those with primary immunodeficiency (PI). Early on, IDF realized that no one was collecting information directly from those impacted with PI as a group of rare diseases. As such, I was brought on to help routinize the collections of your experiences to make sure that the “patient voice” plays a prominent part in the research conducted in PI. This “patient voice” is often referred to as patient-reported data or patient-reported outcomes.
For more than 14 years, this has been my main mission at IDF. With the help of talented IDF staff, collaborations with brilliant and dedicated immunologist researchers, particularly those with PI who participate in our research efforts, USIDNET, surveys, PI CONNECT, and IDF Research Grants, we have been successful in fulfilling that mission.
Our research has resulted in dozens of publications in peer-reviewed journals, which themselves have been cited by others hundreds of times! This is an incredibly important way to improve the medical literature on PI and drive advances and improvements in the field. The ultimate results of our efforts are the improvements in the quality of life for those affected by PI.
A lot has changed in 14 years in research. The movement has been toward focusing on patients and the patient experience as a valuable and integral part of the science. Once seen as an almost afterthought, patients are starting to take their rightful place at the center of research being performed today.
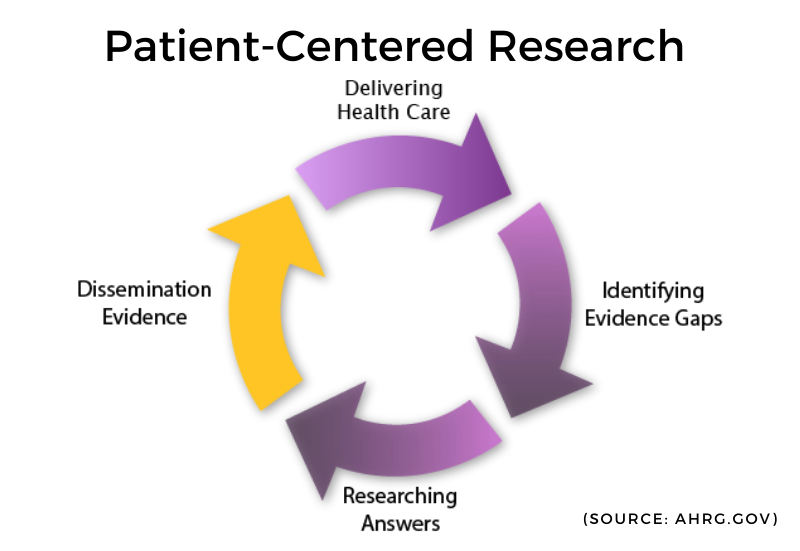
How we got here
I remember sitting at my desk in the old IDF offices, overlooking Chesapeake Ave in downtown Towson, MD. My phone rang, and as most people do, I screened the call by looking at the caller ID. The name and phone number told me it was Dr. Charlotte Cunningham-Rundles. Dr. Cunningham-Rundles is one of the giants in the world of immunology. With all of her accomplishments and dedication to those with PI and to IDF, she is NOT someone who deserves to be screened, so I jumped at my telephone handset to answer her call. After our initial pleasantries, Dr. Cunningham-Rundles, as the Principal Investigator for the United States Immunodeficiency Network (USIDNET), wanted to know if those with X-linked agammaglobulinemia (XLA) had a greater amount of autoimmune and auto-inflammatory diseases than what was known and recorded in the USIDNET Registry. The idea was simple. Perform a patient survey of those with PI and compare those results and data to what exists in USIDNET. For most of the readers here, it is probably not surprising that we found survey respondents reported significantly higher incidences of autoimmune and auto-inflammatory conditions than what existed in the USIDNET registry. This is important as it demonstrates the fact that patients have information about their own conditions and lives that may not exist in clinical registries and in the medical literature. As a matter of fact, we ended up publishing this research in the Journal of Clinical Immunology.
For those of you who may not know about USIDNET, it is a research consortium established to advance scientific research in the field of primary immunodeficiency (PI) by assembling and maintaining a registry of clinical data from patients with PI, providing education and mentoring for young investigators and physicians in the field of PI, and acting as a resource for clinical and laboratory research.
A program of the Immune Deficiency Foundation (IDF), USIDNET is funded by a cooperative agreement from the National Institute of Allergy and Infectious Diseases (NIAID) and the National Institutes of Health (NIH). As a national patient-consented registry, it is designed to obtain longitudinal data on many individuals with known or suspected PI. By putting information about individuals with PI from many places into a single registry, we gain knowledge about the rate of occurrence, causes, natural history, and outcomes of PI. IDF started this registry in 1992 specifically to collect data on chronic granulomatous disease (CGD). Ever since, it has slowly expanded to encompass 38 different PI diagnoses and almost 5,500 individuals with PI. Data from USIDNET has been used to help publish more than 60 different peer-reviewed journal articles in that time.
There are strengths and weaknesses to both clinical data and patient-reported outcomes. Finding a way to combine each of these can result in insights and richness of data and information that would otherwise not be possible. Generally, this concept is known as Patient-Centered Research (PCOR). PCOR compares the impact of two or more preventative, diagnostic, treatment, or healthcare delivery approaches on health outcomes, including meaningful to patients. The greater idea is for this process not to be static but continuous and ongoing. Dissemination of this research is central to PCOR. It is informing researchers, healthcare providers, and patients alike.
IDF has always believed in this concept, long before there even was a term known as PCOR. This belief was the basis for IDF’s successful grant application to the Patient-Centered Outcomes Research Institute (PCORI). The idea was to combine the patient-reported data and outcomes found in the IDF electronic personal health record (IDF ePHR) with the clinical data as found in the USIDNET Registry. Our proof of concept and certainty that this was the right track came from our XLA survey/research collaboration experience with USIDNET.
PCORI itself was made up of almost two dozen patient organizations, similar to IDF and about a dozen large commercial healthcare systems - hospital systems or consortia of hospital systems. PCORI did not have a lot of time to demonstrate value and results. Despite excellent collaboration, ideas, and will, there was just not enough time for a single, big-data result to come out of everyone’s determined efforts. Time was not on our side. As a result, after two relatively short phases, funding for PCORI sunset.
IDF was proud to be have been awarded grants for the first two phases of PCORI. IDF helped pioneer a much simpler, more refined, and easy-to-understand online consent process into PI CONNECT/USIDNET. We used our learnings and resources through PI CONNECT to improve both the ePHR and USIDNET. PI CONNECT was even the genesis for the IDF Fever Study. IDF is still committed to the principles and ideas/ideals of PCOR.
Not long after our PCORI grant ended, IDF found out that government funding for USIDNET would end in March 2021. Quite honestly, this was a bit of a shock to IDF staff and the USIDNET Steering Committee. Twenty-six years of a registry in PI seemed to be coming to an end. So much great work had been done in this time, and we all know much remains. We performed this work in 1992 with an early concept of what a registry is, how it should be managed and what it should be. If IDF were to create a registry in PI today, it would be vastly different than the registry we created 26 years ago.
Genomics, computer technology/software, data storage, data integrations, and “big data” are all things that were not part of anyone’s vocabulary in 1993. Even things like the models of how registries are funded and sustained have changed.
Building a new registry for the 21st Century alone, from the ground up, is a Herculean task, demanding massive resources in staff, funds, and time. It is not without risks. We know. We have done the research, and we have the experience. We have been part of national efforts toward this end. We have even presented at patient registry conferences and health information technology conferences based on our experiences and vision for what the future should be.
This is a perfect time and opportunity for IDF to pivot, and reimagine - together - how we can best serve research in PI. Certainly, IDF is committed to preserving the data and incredible legacy of USIDNET 1.0. and PI CONNECT 1.0. The question is, how do we move forward with our community in a way that is the most effective and efficient? One that makes sense and is sustainable over 26 or more additional years?
One of IDF’s strengths is our ability to foster collaboration and to continually advocate for solutions that will improve the lives of those impacted by PI. IDF sits at a unique intersection of those with PI, researchers/immunologist/healthcare providers, the pharmaceutical/biotherapy industry, and other rare disease patient groups and consortia. Over the course of four decades, IDF has developed trust and excellent relationships with all of these groups.
At this juncture, it makes more sense for us to work with all of these strategic partners to further our goals for registries and research in PI. We will leverage IDF’s strengths and assets and work from within to combine efforts in existing registries in rare disease, clinical research, clinical trials, and post-market drug/therapy studies. Important keys to that effort are to
- continually raise “the patient voice” and perspective in these areas
- break down existing “data silos”
- demand more transparency and sharing/dissemination of results that matter to those with PI
- fight for and find ways for research to be more inclusive, diverse, and accessible
One thing the last 18 months of this pandemic has taught me is that we need to be flexible and adapt to new challenges as circumstances change and new opportunities arise. When one window closes, another window or even door opens. We need to look for, find these new openings and use them to advance our cause. That is exactly what IDF is doing. We have already found these windows of opportunity and are in the process of making the most of them. Some are going to work out; some will not. That is the nature of research. That is the nature of life.
A few parting thoughts for this month
At this very moment, IDF is helping shape the future of research in PI. There are a lot of exciting projects coming: a few surveys, some opportunities for researchers to learn from your experiences, and yes, even a new registry.
Our research efforts cannot be successful without you. Individually and as a community, IDF needs your active participation in this process. Without it, we fail.
So, the next time you see an IDF Survey come across in an email, or read about a new clinical trial in PI (from IDF or others), or have an opportunity to be part of a registry (from IDF or others), please take the time to stop, read and consider the fact that when you as an individual choose to participate, you make a difference. A difference your community needs.
Thank you for indulging me in the first of what I hope are many research-related blog posts. In the meantime, please feel free to comment on anything you have read here. Give me your thoughts and ideas. Let’s keep the conversation going!
Stay healthy, stay involved!
ABOUT THE AUTHOR:
Reflections on Research is a monthly column written by Christopher Scalchunes, MPA. Christopher is the Vice President of Research at the Immune Deficiency Foundation, where he has worked since 2007. While he is neither an immunologist nor a medical doctor, he does have an extensive background in public policy and survey research. He is a husband and proud father of three who loves cooking, hiking, and animals.
Topics
Sign up for updates from IDF
Receive news and helpful resources to your cell phone or inbox. You can change or cancel your subscription at any time.





The Immune Deficiency Foundation improves the diagnosis, treatment, and quality of life for every person affected by primary immunodeficiency.
We foster a community that is connected, engaged, and empowered through advocacy, education, and research.
Combined Charity Campaign | CFC# 66309




