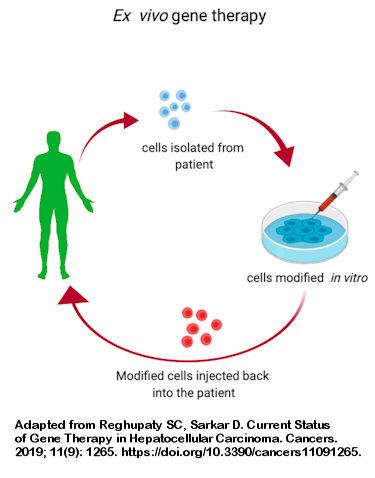
History of gene therapy to treat PI
Gene therapy has been used to treat individuals with several types of severe combined immunodeficiency (SCID), hereditary angioedema (HAE) type 1, X-linked chronic granulomatous disease (X-linked CGD), leukocyte adhesion deficiency-1 (LAD-1), and WAS.
Gene therapy for ADA-SCID
The first clinical trial for gene therapy was at the National Institutes of Health in 1990 and treated a 4-year-old girl with ADA-SCID. The design of this first trial did not attempt to correct her stem cells, only her T cells. This patient is still clinically well and has about 25% of her circulating T cells carrying the corrected ADA gene more than 30 years after her treatment.
After this initial clinical trial demonstrated that gene therapy could be carried out safely and that gene-corrected T cells could survive for years and function normally, follow-up trials were initiated attempting to treat other children with ADA-SCID by targeting stem cells from the bone marrow. In terms of providing a functioning immune system, the results have been excellent, with most of the nearly 100 individuals treated for ADA-SCID attaining a significant, long-lasting increase in T and B cell counts and a remarkable improvement in immune function.
In 2016, the European Medicines Agency (EMA) approved an ADA-SCID gene therapy product called Strimvelis that uses a gammaretroviral vector. Thirty-three patients were treated with Strimvelis before reports in the fall of 2020 that one patient developed leukemia four years after treatment due to insertional oncogenesis. Insertional oncogenesis is a known risk with gammaretroviral vectors. These vectors carry DNA sequences that can activate other genes near where the new functional gene is integrated into the stem cell’s chromosome and, depending on which genes are nearby, can make a stem cell more likely to become cancerous. EMA issued a direct healthcare professional communication in early 2021 advising clinicians to follow patients closely for signs of leukemia. Strimvelis remains authorized in the European Union.
An ongoing clinical trial for ADA-SCID gene therapy using a different viral vector is active in the U.S. at the University of California, Los Angeles.
Gene therapy for other PIs
The first gene therapy trials for X-linked SCID, X-linked CGD, and WAS also used gammaretroviral vectors to introduce functional versions of the affected genes into individuals' hematopoietic stem cells. While these trials resulted in restored immune function for the majority of treated individuals, a substantial number of them went on to develop leukemia in the years following treatment due to insertional oncogenesis caused by the viral vector.
More recent trials for several types of PI have used viral vectors designed to avoid the risk of insertional oncogenesis and have led to similar, if not better, immune benefits as in the early trials. No cases of leukemia have yet been tied to any gene therapy using a non-gammaretroviral vector.