
The immune system and genetics
Primary immunodeficiencies can be inherited in one of three different ways depending on the specific disorder: X-linked recessive, autosomal recessive, or autosomal dominant.

The more you understand about primary immunodeficiency (PI), the better you can live with the disease or support others in your life with PI. Learn more about PI, including the various diagnoses and treatment options.

Living with primary immunodeficiency (PI) can be challenging, but you’re not alone—many people with PI lead full and active lives. With the right support and resources, you can, too.

Be a hero for those with PI. Change lives by promoting primary immunodeficiency (PI) awareness and taking action in your community through advocacy, donating, volunteering, or fundraising.

Whether you’re a clinician, researcher, or an individual with primary immunodeficiency (PI), IDF has resources to help you advance the field. Get details on surveys, grants, and clinical trials.

Primary immunodeficiencies can be inherited in one of three different ways depending on the specific disorder: X-linked recessive, autosomal recessive, or autosomal dominant.
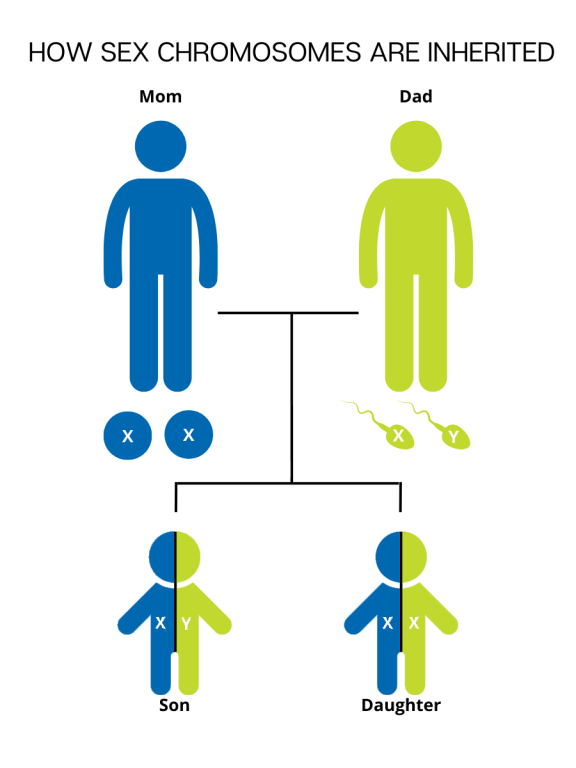
Each cell in the human body contains genetic material that carries the instructions for every protein, cell, and organ that makes up the body. This "master plan" genetic material is packaged into 23 pairs of chromosomes, for 46 total chromosomes. There are 22 pairs of numbered chromosomes (also known as autosomes), and one pair of sex chromosomes (XX for females and XY for males). Children inherit one chromosome in each pair from their mother and one chromosome in each pair from their father.

During fertilization, the egg, which contains 23 single chromosomes, fuses with the sperm, which also contains 23 single chromosomes, and the resulting fetus has 46 total chromosomes. This way, each parent contributes half of the genetic information for their child. The sex of the child is determined by which sex chromosome (X or Y) the sperm that fuses with the egg (only X) carries. An X chromosome from the sperm results in a female offspring and a Y chromosome from the sperm results in a male offspring.
The genetic material packaged in these chromosomes is made up of deoxyribonucleic acid (DNA), which is composed of individual molecules called nucleotides. Four nucleotides make up DNA: adenine (A), thymidine (T), guanine (G), and cytosine (C). The order of these nucleotides, or the DNA "sequence," encodes information in long chains that are arranged in a specific way, similar to the arrangement of letters to form words and sentences.
Variants in the spelling of the words (because of misplacement of one or more nucleotides) lead to genetic differences between people. Some spelling variants might not lead to a significant change in the genetic instructions, and those variants do not cause disease. Other spelling variants do significantly change the instructions, and those types of variants can cause diseases such as PI.
If these gene variants are passed down in the egg or sperm of one or both parents, they can cause disease in the child. Occasionally, variants spontaneously occur in a fertilized egg, and neither of the parents actually carry the variant.
Download this chapter from the IDF Patient & Family Handbook for Primary Immunodeficiency Diseases, Sixth Edition.
Download PDFPI can be inherited in one of three different ways: autosomal recessive, autosomal dominant, or X-linked recessive. Family history and genetic testing can be helpful in establishing the possible role of genes or chromosomes in a particular PI and may be useful to identify a particular pattern of inheritance.
Genes present on one of the 22 pairs of numbered chromosomes are known as autosomal. In autosomal recessive inheritance, two copies of the PI-causing gene variant must be inherited to cause symptoms of the condition, typically one from each parent.
Usually, each parent of the child affected by an autosomal recessive condition carries one copy of the PI-causing gene variant, and they are unaffected because their other copy of the gene is functional. In this scenario, where both parents are carriers of an autosomal recessive gene variant, there is a 25% chance (1 in 4) that any child, regardless of gender, will be affected by the disorder. There is a 50% chance (1 in 2) that any child will be a carrier (inherit one copy of the variant), and a 25% (1 in 4) that the child will not inherit the at all, and therefore will not be affected by the condition or be able to pass it on to their children.
The chances of a child being affected, a carrier, or neither remain the same for all future pregnancies. The outcome of each pregnancy is independent and is not affected by previous pregnancies.
Examples of autosomal recessive PIs:
Autosomal recessive PIs are rare. Most affected infants do not have any previous family history. However, marriages between people who are related by blood or increased frequency of specific variants in certain populations (such as the Artemis SCID variant in Navajo populations) increase the incidence of such conditions.
Autosomal dominant inheritance is a mode of inheritance where only one copy of the PI-causing gene variant is needed for the disease to occur, regardless of the version of the gene inherited from the other parent. Both males and females can be affected, and there is a 50% chance (1 in 2) of the child of an affected parent inheriting the disease. The risk is the same in every pregnancy.
Sometimes, a patient affected with an autosomal dominant condition will be the first affected person in their family. In this case, the variant spontaneously occurs in the fertilized egg after the joining of egg and sperm.
Examples of autosomal dominant PIs:
X-linked recessive is a mode of inheritance where the gene variant causing PI is present on the X chromosome. Because males have only one X chromosome, if that X chromosome carries the gene variant, the individual will be affected by the PI. Males have no additional X chromosome to compensate and the Y chromosome does not carry the same set of genes as the X chromosome.
Females have two X chromosomes and must inherit two copies of the gene variant—one from each parent—to develop the disorder. Females are carriers if they inherit one variant because the second copy of the X-linked gene continues to carry out its function. Carriers can pass on the gene variant to their children.
An affected male will pass on the gene variant to 100% of his female children because they will all inherit his X chromosome. In most cases, his daughters will be carriers, unless their mother also happens to be a carrier—then, there is a 50% chance of each daughter being affected.
All of an affected male’s sons will carry the Y chromosome and will therefore neither be affected nor be carriers.
At times, female carriers of X-linked disorders do present with symptoms similar to affected males due to a process called X-inactivation. In this process, which happens during fetal development, some cells shut down (inactivate) the X chromosome inherited from the mother, and other cells inactivate the X chromosome inherited from the father. Which X chromosome each cell inactivates is random.
In the case of an X-linked carrier, if the inactivation happened to the X chromosome coming from the mother, then the only active chromosome will be the X chromosome coming from the father, which carries the disease-causing variant, leading to symptoms similar to the affected males.
Mothers who are carriers can pass on the disease to their sons if the sons inherit the affected X. In this type of inheritance, a family history may show multiple males affected by the disease. Mothers who are carriers can also pass on the affected X to their daughters, who then become carriers of the disease themselves.
Variants in X chromosome genes may also occur spontaneously and may occur in individuals with no previous family history.
Examples of X-linked recessive PIs:
The chances of a woman who carries a PI-causing X-linked gene variant having an affected child are different based on the gender of the child. If the father is not affected, there are four possible combinations in every pregnancy.
The outcome of future pregnancies is not affected by previous pregnancies, meaning that it is possible for all (100%) of the boys in a family where the mother is a carrier to be affected by the X-linked condition.
This page contains general medical and/or legal information that cannot be applied safely to any individual case. Medical and/or legal knowledge and practice can change rapidly. Therefore, this page should not be used as a substitute for professional medical and/or legal advice. Additionally, links to other resources and websites are shared for informational purposes only and should not be considered an endorsement by the Immune Deficiency Foundation.
Adapted from the IDF Patient & Family Handbook for Primary Immunodeficiency Diseases, Sixth Edition.
Copyright ©2019 by Immune Deficiency Foundation, USA
Receive news and helpful resources to your cell phone or inbox. You can change or cancel your subscription at any time.





The Immune Deficiency Foundation improves the diagnosis, treatment, and quality of life for every person affected by primary immunodeficiency.
We foster a community that is connected, engaged, and empowered through advocacy, education, and research.
Combined Charity Campaign | CFC# 66309
