
-
Understanding primary immunodeficiency (PI)

Understanding PI
The more you understand about primary immunodeficiency (PI), the better you can live with the disease or support others in your life with PI. Learn more about PI, including the various diagnoses and treatment options.
-
Living with PI
-
Addressing mental health
-
Explaining your diagnosis
- General care
- Get support
- For parents and guardians
-
Managing workplace issues
- Navigating insurance
-
Traveling safely

Living with PI
Living with primary immunodeficiency (PI) can be challenging, but you’re not alone—many people with PI lead full and active lives. With the right support and resources, you can, too.
-
Addressing mental health
-
Get involved

Get involved
Be a hero for those with PI. Change lives by promoting primary immunodeficiency (PI) awareness and taking action in your community through advocacy, donating, volunteering, or fundraising.
-
Advancing research and clinical care
-
Research Grant Program
-
Consulting immunologist
-
Diagnosing PI
-
Getting prior authorization
-
Clinician education
-
Survey research
-
Participating in clinical trials

Advancing research and clinical care
Whether you’re a clinician, researcher, or an individual with primary immunodeficiency (PI), IDF has resources to help you advance the field. Get details on surveys, grants, and clinical trials.
-
Research Grant Program
Mutations in genes of persons with chronic granulomatous disease (CGD) affect the person’s production of superoxide, which in turn dictates the severity of CGD symptoms, according to a review of CGD by Dr. Jennifer Leiding and Dr. Steven Holland for the National Institute of Health’s National Library of Medicine.
The six genes that can be involved in CGD are:
CYBB
CYBA
CYBC1
NCF1
NCF2
NCF4
Each of these genes is responsible for the function of a different part of an enzyme that helps immune cells called phagocytes make superoxide. Superoxide kills certain bacteria and fungi.
When any one of the aforementioned genes is mutated, it results in low or no production of superoxide, making a person vulnerable to infection by bacteria and fungi, and causing inflammation in the body. The less superoxide a person makes, the more at risk the person is for infection. The more superoxide a person makes, the greater the protection from infection.
CGD can be inherited through an X-linked or autosomal recessive pattern. Mutations in the CYBB gene cause X-linked CGD, which means a son inherits the condition from his mother if she is a carrier. X-linked CGD is the most prevalent type of CGD. Mutations in the CYBA, CYBC1, NCF1, NCF2, and NCF4 genes cause autosomal recessive types of CGD, which means a son or daughter inherits the condition from both parents if they are carriers. Carriers are also sometimes affected by the condition.
Persons with CGD experience the following symptoms:
Infections of the lung, lymph nodes, liver, bone, and skin
Delayed growth
Granuloma (inflammation of a small area) formation
Colitis (inflammation of the colon)
Poor wound healing
Certain genetic mutations associated with X-linked CGD cause more significant, life-threatening health problems than mutations found in autosomal recessive CGD because some mutations in X-linked CGD result in no superoxide production, greatly reducing a person’s ability to fight infection.
“Historically, it has been recognized that pathogenic variants in CYBB (the cause of X-linked CGD) give rise to a more serious phenotype than pathogenic variants causing autosomal recessive forms of CGD,” according to the review.
“Compared to persons with autosomal recessive CGD, males with X-linked CGD are typically diagnosed earlier and have… higher mortality at a young age.”
However, CGD characterized by residual superoxide production, found in autosomal recessive CGD, and a form of CGD called protein-positive X-linked CGD, “typically have a milder course and come to clinical attention later in life than those with absent protein expression,” according to the review.
In addition, the review states, “CYBC1 and NCF4 are notable for severe and difficult-to-control inflammatory bowel disease, with relatively less common severe infections.”
CGD is diagnosed through blood tests that measure the production of superoxide and other chemicals. Follow-up molecular genetic testing aids in identifying the gene responsible for CGD.
To prevent infection, those with CGD take prophylactic lifelong daily antibiotics and antifungals. Another prophylactic treatment is gamma interferon, which stimulates superoxide production, and requires injections three times a week. One study showed a 70 percent reduction in infections in gamma interferon recipients, and it has become part of the prophylactic regimen in most centers in the U.S., according to the review.
For those with more severe forms of CGD, a hematopoietic stem cell transplant (HSCT) is a treatment option. HSCT, also known as bone marrow transplant (BMT), uses stem cells from a donor to replace the stem cells in the recipient, providing a person with CGD with a functioning immune system.
Though HSCT used to be “reluctantly offered” because it led to higher levels of long-term illness and mortality, the use of lower and less toxic doses of chemotherapy to prepare recipients for the transplant has reduced toxicity and has allowed for a transplant even if the recipient has an active infection, according to the review.
“Recent reports show excellent overall survival and event-free survival, especially when HSCT is performed with matched donors and at a younger age,” according to the review.
Gene therapy clinical trials also offer treatment for CGD.
Related resources
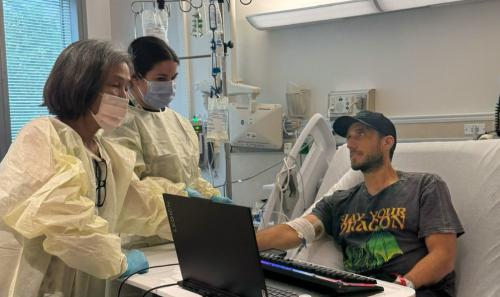
Man with X-linked hyper IgM first-ever to receive novel gene therapy
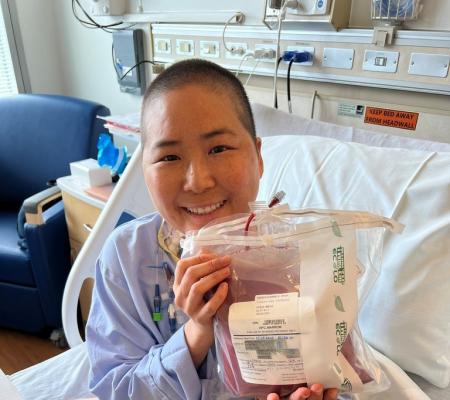
Pharmacist with CVID receives bone marrow transplant

Undiagnosed: Reuben & Sherri Johnson on CGD, chronic illness, and the fight for healthcare
Sign up for updates from IDF
Receive news and helpful resources to your cell phone or inbox. You can change or cancel your subscription at any time.





The Immune Deficiency Foundation improves the diagnosis, treatment, and quality of life for every person affected by primary immunodeficiency.
We foster a community that is connected, engaged, and empowered through advocacy, education, and research.
Combined Charity Campaign | CFC# 66309

