-
Understanding primary immunodeficiency (PI)

Understanding PI
The more you understand about primary immunodeficiency (PI), the better you can live with the disease or support others in your life with PI. Learn more about PI, including the various diagnoses and treatment options.
-
Living with PI
-
Addressing mental health
-
Explaining your diagnosis
- General care
- Get support
- For parents and guardians
-
Managing workplace issues
- Navigating insurance
-
Traveling safely

Living with PI
Living with primary immunodeficiency (PI) can be challenging, but you’re not alone—many people with PI lead full and active lives. With the right support and resources, you can, too.
-
Addressing mental health
-
Get involved

Get involved
Be a hero for those with PI. Change lives by promoting primary immunodeficiency (PI) awareness and taking action in your community through advocacy, donating, volunteering, or fundraising.
-
Advancing research and clinical care
-
Research Grant Program
-
Consulting immunologist
-
Diagnosing PI
-
Getting prior authorization
-
Clinician education
-
Survey research
-
Participating in clinical trials

Advancing research and clinical care
Whether you’re a clinician, researcher, or an individual with primary immunodeficiency (PI), IDF has resources to help you advance the field. Get details on surveys, grants, and clinical trials.
-
Research Grant Program

As the IDF Research Grant Program applicant with the highest-scoring application, this year’s Michael Blaese Research Grant Award goes to Dr. Donald Kohn, distinguished professor at the University of California, Los Angeles (UCLA), and director of the UCLA David Geffen School of Medicine Human Gene and Cell Therapy Program. Kohn's name may be familiar, as he has devoted his career to developing gene therapy for PIs, including adenosine deaminase deficient severe combined immunodeficiency (ADA-SCID) and leukocyte adhesion deficiency-I (LAD-1). IDF’s grant will help him move that work into the age of gene editing for a PI that has been difficult to address with traditional gene therapy—X-linked agammaglobulinemia (XLA).
In his previous work, Kohn introduced a working copy of a gene into patients’ blood-forming, or hematopoietic, stem cells using a type of gene therapy vector that inserts the gene at somewhat random sites in the cells’ DNA. That works well for genes like adenosine deaminase that don’t require tight control over where and when they are turned on versus off.
In contrast, Kohn said, “Mother Nature controls the expression of [the BTK] gene very precisely,” referencing the gene, Bruton's tyrosine kinase (BTK), that is disrupted in XLA. Typically, such tight control relies on the DNA surrounding a gene. If a tightly controlled gene is inserted in another place and loses its DNA context, it can cause problems. In fact, using a traditional vector to introduce BTK in mice causes abnormal cell growth and division of white blood cells, which can lead to cancer.
For this reason, Kohn has been working on a more precise approach for XLA. Instead of just plopping a working copy of BTK down somewhere in cells’ DNA, he is using gene editing with CRISPR/Cas9 to insert BTK just in front of the nonworking copy in each cell. Kohn’s lab has already shown that the approach works in human cell lines and non-XLA stem cells without any troubling side effects.
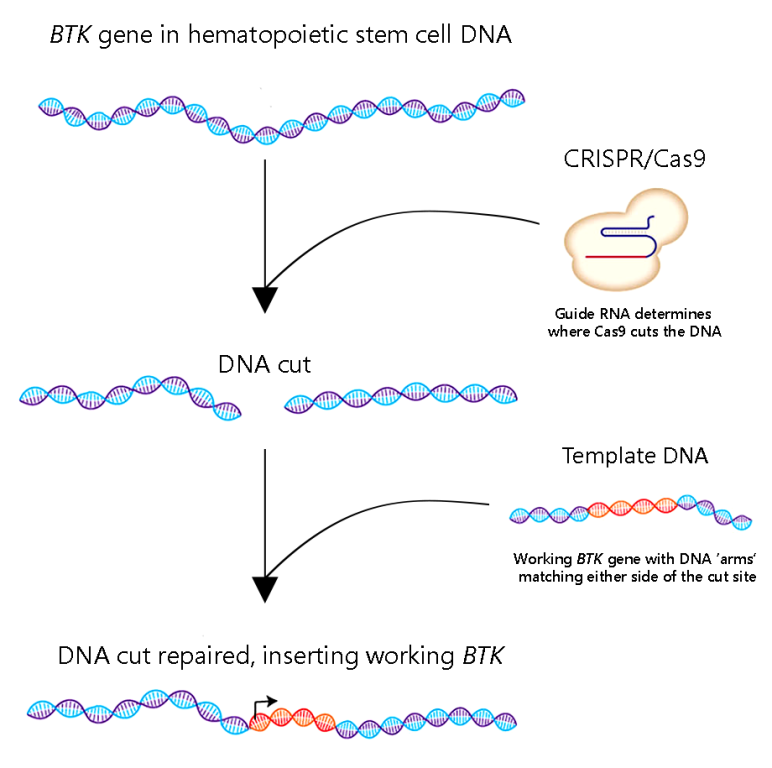
Kohn plans to use the IDF grant to gather additional pre-clinical data he hopes will dovetail with ongoing experiments in mice—testing the BTK gene editing strategy in hematopoietic stem cells from individuals with XLA.
Not only will Kohn test the ability of these edited cells to make the BTK protein, he also plans to infuse the edited cells into a mouse model of XLA. “If we can show they grow as far as normal donors’ [cells] do in those models, and show the mouse model parallel approach leads to vaccine response, we think that’ll be good evidence to get to clinical trial,” he said. He hopes to start clinical trials in two to four years if everything goes well.
Kohn’s also thinking about how to get a therapy like this to patients following setbacks over the past year in the gene therapy commercial market.
“Is commercialization by pharma the only model? Or can, for example, [the therapy] continue to be made at a university but reimbursed by insurance?” he wondered. “It’s getting so that the science isn’t what’s limiting. The financial, the regulatory [issues] are becoming the rate-limiting issues.”
In discussing his IDF-supported research, Kohn remarked that he was especially honored to be named the Blaese awardee. “I started as a fellow in Mike’s lab. I joined Mike in 1985, and one of the first things he said to me was ‘How would you like to work on gene therapy for your fellowship?’ And I thought, wow, that sounds cool,” Kohn said. “It’s always with me, the lessons I learned with him, so it’s really nice to get this award in his name.”
This page contains general medical and/or legal information that cannot be applied safely to any individual case. Medical and/or legal knowledge and practice can change rapidly. Therefore, this page should not be used as a substitute for professional medical and/or legal advice. Additionally, links to other resources and websites are shared for informational purposes only and should not be considered an endorsement by the Immune Deficiency Foundation.
Related resources
Sign up for updates from IDF
Receive news and helpful resources to your cell phone or inbox. You can change or cancel your subscription at any time.





The Immune Deficiency Foundation improves the diagnosis, treatment, and quality of life for every person affected by primary immunodeficiency.
We foster a community that is connected, engaged, and empowered through advocacy, education, and research.
Combined Charity Campaign | CFC# 66309