-
Understanding primary immunodeficiency (PI)

Understanding PI
The more you understand about primary immunodeficiency (PI), the better you can manage it. Learn about PI diagnoses and treatment options.
-
Living with PI
-
Addressing mental health
-
Explaining your diagnosis
- General care
- Get support
- For parents and guardians
-
Managing workplace issues
- Navigating insurance
-
Traveling safely

Living with PI
Living with primary immunodeficiency (PI) can be challenging, but you’re not alone—many people with PI lead full and active lives. With the right support and resources, you can, too.
-
Addressing mental health
-
Get involved

Get involved
Be a hero for those with PI. Change lives by promoting primary immunodeficiency (PI) awareness and taking action in your community through advocacy, donating, volunteering, or fundraising.
-
Advancing research and clinical care
-
Research Grant Program
-
Consulting immunologist
-
Diagnosing PI
-
Getting prior authorization
-
Clinician education
-
Survey research
-
Participating in clinical trials

Advancing research and clinical care
Whether you’re a clinician, researcher, or an individual with primary immunodeficiency (PI), IDF has resources to help you advance the field. Get details on surveys, grants, and clinical trials.
-
Research Grant Program
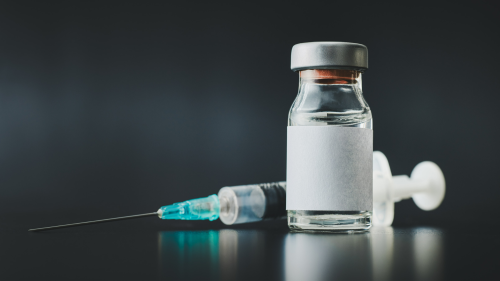
The U.S. Food and Drug Administration (FDA) has issued its first emergency use authorization (EUA) for a monoclonal antibody (mAb) to prevent COVID-19 since Evusheld’s EUA was pulled in early 2023. Pemivibart, marketed under the trade name Pemgarda, is made by Invivyd and is meant to be used before exposure to prevent COVID-19 in those who are at least 12 years of age, weigh at least 88 pounds, and are moderately to severely immunocompromised.
“COVID-19 continues to pose a significant threat and major concern to those who are moderately to severely immunocompromised,” said Jorey Berry, President and CEO of the Immune Deficiency Foundation (IDF) and a steering committee member of the Immunocompromised Collaborative. “As such, we are delighted that a new monoclonal antibody for pre-exposure prophylaxis of COVID-19 will be available soon for certain vulnerable populations.”
Many people who are immunocompromised, including some with primary immunodeficiencies (PIs), do not respond to vaccines. COVID-19 vaccines are active immunizations that require the recipient’s immune system to generate protective antibodies, whereas Pemgarda provides passive protection no matter the status of a person’s immune system. The FDA stressed that Pemgarda is not meant to be a substitute for vaccination against COVID-19 and that only those who are likely to have a poor response to vaccines should receive it. Pemgarda is also not authorized to treat COVID-19 or to act preventatively once someone has been exposed to COVID-19.
The mAb is given as a single intravenous (IV) infusion in a healthcare setting equipped to monitor recipients for adverse reactions such as anaphylaxis. Like Evusheld, Pemgarda has been modified to extend the life of the antibody in a person’s bloodstream but will need to be re-dosed every three months to maintain protection.
Invivyd pursued a unique ‘immunobridging’ strategy to gain authorization for Pemgarda from the FDA. Prior to the rise of the Omicron variant, Invivyd developed a mAb meant to compete with AstraZeneca’s Evusheld. However, midway through clinical trials, laboratory experiments showed that Invivyd’s mAb could not neutralize the quickly rising Omicron variant. Evusheld’s EUA was revoked because of its lack of activity against Omicron as well. In talks with the FDA, Invivyd argued that data from those trials could be used to support Pemgarda’s authorization since it is derived from that mAb candidate, uses the same manufacturing platform, and is functionally similar. The FDA agreed, significantly shortening the development process for Pemgarda.
Invivyd continues to monitor Pemgarda’s neutralizing activity against new COVID-19 variants as they arise and has shown that it maintains activity against JN.1, which is currently the dominant variant circulating in the U.S.
Screen documentary
Host a private screening of Compromised: Life Without Immunity to experience the resilience, hope, and determination of the PI community. And know that your hosted event can be two people or 100+!
Watch the filmThis page contains general medical and/or legal information that cannot be applied safely to any individual case. Medical and/or legal knowledge and practice can change rapidly. Therefore, this page should not be used as a substitute for professional medical and/or legal advice. Additionally, links to other resources and websites are shared for informational purposes only and should not be considered an endorsement by the Immune Deficiency Foundation.
Related resources
Sign up for updates from IDF
Receive news and helpful resources to your cell phone or inbox. You can change or cancel your subscription at any time.




The Immune Deficiency Foundation improves the diagnosis, treatment, and quality of life for every person affected by primary immunodeficiency.
We foster a community that is connected, engaged, and empowered through advocacy, education, and research.