
-
Understanding primary immunodeficiency (PI)

Understanding PI
The more you understand about primary immunodeficiency (PI), the better you can live with the disease or support others in your life with PI. Learn more about PI, including the various diagnoses and treatment options.
-
Living with PI
-
Addressing mental health
-
Explaining your diagnosis
- General care
- Get support
- For parents and guardians
-
Managing workplace issues
- Navigating insurance
-
Traveling safely

Living with PI
Living with primary immunodeficiency (PI) can be challenging, but you’re not alone—many people with PI lead full and active lives. With the right support and resources, you can, too.
-
Addressing mental health
-
Get involved

Get involved
Be a hero for those with PI. Change lives by promoting primary immunodeficiency (PI) awareness and taking action in your community through advocacy, donating, volunteering, or fundraising.
-
Advancing research and clinical care
-
Research Grant Program
-
Consulting immunologist
-
Diagnosing PI
-
Getting prior authorization
-
Clinician education
-
Survey research
-
Participating in clinical trials

Advancing research and clinical care
Whether you’re a clinician, researcher, or an individual with primary immunodeficiency (PI), IDF has resources to help you advance the field. Get details on surveys, grants, and clinical trials.
-
Research Grant Program
On March 24, 2023, the U.S. Food and Drug Administration (FDA) approved leniolisib (trademarked as Joenja by Pharming) for the treatment of activated PI3K delta syndrome (APDS; also known as PASLI disease). The medication is the first drug approved for the treatment of APDS, an ultrarare primary immunodeficiency (PI) estimated to affect one in 1–2 million people worldwide. Leniolisib’s approval shows how understanding exactly how PI-causing genetic variants affect cells and lead to symptoms can spur the development of precise and effective treatments.
APDS was first described in 2013 and is a combined immunodeficiency, affecting both B cells and T cells. Individuals with APDS experience frequent respiratory tract infections that can lead to lung damage (bronchiectasis), chronic infection with herpesviruses (for example, Epstein-Barr virus), increased generation of white blood cells (lymphoproliferation), enlarged lymph nodes, spleen, and/or liver, inflammation of the gut, and low numbers of circulating blood cells (cytopenia). As people with APDS enter adulthood, their previously harmless lymphoproliferation tends to progress to lymphoma, a type of blood cancer.
There are two types of APDS, APDS 1 and APDS 2, caused by genetic variants in the PIK3CD or PIK3R1 genes, respectively. Both types are inherited as autosomal dominant disorders.
In white blood cells, the proteins coded for by the PIK3CD and PIK3R1 genes come together to form an enzyme that is turned on when receptor proteins on the surface of these cells detect an infection. When the enzyme, called PI3Kδ, is turned on, it ultimately causes B and T cells to divide and become active. In people without APDS, PI3Kδ is very carefully controlled and only gets turned on when in response to an infection. But in people with APDS, the genetic variant in the PIK3CD or PIK3R1 gene causes PI3Kδ to be turned on all the time.
While you might expect people with APDS to have an overactive immune response rather than an immunodeficiency, the human immune system is complicated. Having PI3Kδ active all the time throws off the delicate balance of all of the different types of B and T cells that the immune system needs to function. There are too many mature, activated T cells and not enough naive T cells. Similarly, there are too many immature transitional B cells and fully developed plasma cells making IgM antibodies, and not enough of other B cell types. In addition, B cells that produce autoantibodies survive when they are normally weeded out. The result is ineffective antibody and T cell responses to infections, even though B and T cells are growing and dividing all the time, plus a side of autoimmunity.
To date, APDS has been treated with preventative antibiotics, immunoglobulin replacement therapy, immune-suppressing drugs, and hematopoietic stem cell transplant (HSCT), depending on an individual’s clinical symptoms. However, because B cell cancers also have overactive PI3Kδ, scientists have been working on drugs that block the enzyme’s activity for a while. Leniolisib was synthesized in 2017 by scientists at Novartis looking for a drug that worked specifically on PI3Kδ (and not on related enzymes), and that avoided some of the safety issues with earlier PI3Kδ inhibitors.
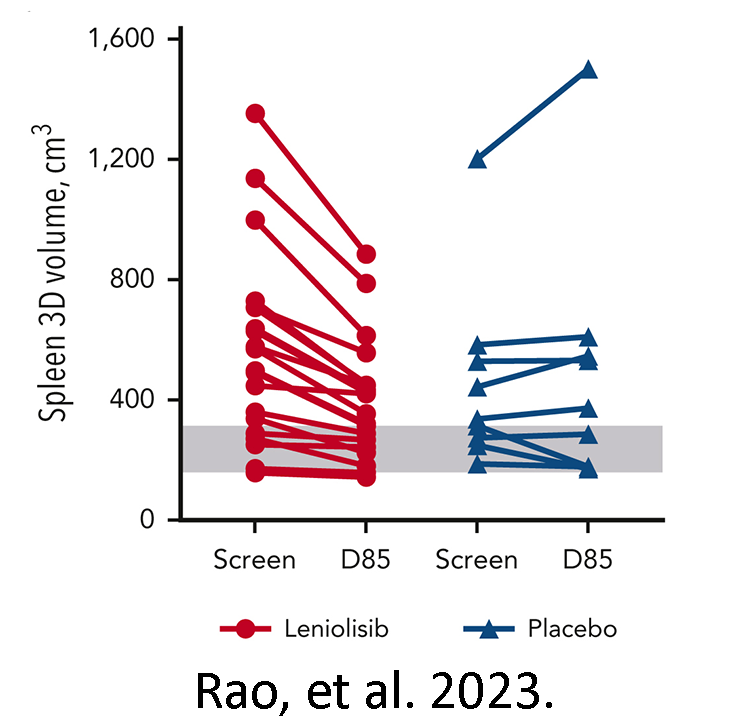
shows spleen size in individuals on leniolisib (red) and
placebo (blue) from before the trial (screen) to day 85 of
the trial (D85). The grey range indicates typical spleen
size for an adult.
Pharming licensed leniolisib from Novartis and continued its clinical development. FDA’s approval of the drug for those at least 12 years of age was based in part on recently published phase III clinical trial results from 31 individuals with either APDS 1 or 2 who took leniolisib as a pill twice daily over 12 weeks. Leniolisib significantly reduced lymph node and spleen/liver size over the placebo, indicating less lymphoproliferation. Those given leniolisib also had more balanced B and T cell populations at the end of the trial than at the beginning, and as compared to those given the placebo. Importantly, no serious side effects from taking leniolisib were reported.
Because of symptom overlap with other PIs, individuals with APDS are sometimes initially diagnosed with CVID, hyper IgM syndrome, or an unknown PI, and typically receive an APDS diagnosis only after genetic testing. Anticipating that leniolisib would ultimately be approved for treating APDS, Pharming has partnered with Invitae to offer free genetic testing for APDS 1 and 2 for certain individuals. Pharming also successfully campaigned for an APDS-specific ICD-10 diagnostic code, which went into effect on October 1, 2022.
Pharming expects to make leniosilib available to those with APDS in the U.S. by mid-April. The drug is also under review by the European Medicines Agency, with a decision expected in the second half of 2023. Since APDS symptoms tend to start in early childhood and progress over time, there are also phase III clinical trials planned or ongoing for those 4–11 years old and 1–6 years old.
Related resources
Sign up for updates from IDF
Receive news and helpful resources to your cell phone or inbox. You can change or cancel your subscription at any time.





The Immune Deficiency Foundation improves the diagnosis, treatment, and quality of life for every person affected by primary immunodeficiency.
We foster a community that is connected, engaged, and empowered through advocacy, education, and research.
Combined Charity Campaign | CFC# 66309




