
-
Understanding primary immunodeficiency (PI)

Understanding PI
The more you understand about primary immunodeficiency (PI), the better you can live with the disease or support others in your life with PI. Learn more about PI, including the various diagnoses and treatment options.
-
Living with PI
-
Addressing mental health
-
Explaining your diagnosis
- General care
- Get support
- For parents and guardians
-
Managing workplace issues
- Navigating insurance
-
Traveling safely

Living with PI
Living with primary immunodeficiency (PI) can be challenging, but you’re not alone—many people with PI lead full and active lives. With the right support and resources, you can, too.
-
Addressing mental health
-
Get involved

Get involved
Be a hero for those with PI. Change lives by promoting primary immunodeficiency (PI) awareness and taking action in your community through advocacy, donating, volunteering, or fundraising.
-
Advancing research and clinical care
-
Grants
-
IDF surveys
-
Participating in clinical trials
-
Diagnosing PI
-
Consulting immunologist
-
Clinician education

Advancing research and clinical care
Whether you’re a clinician, researcher, or an individual with primary immunodeficiency (PI), IDF has resources to help you advance the field. Get details on surveys, grants, and clinical trials.
-
Grants
Update
On September 29, the U.S. Centers for Disease Control and Prevention (CDC) issued a health advisory on severe monkeypox in those who are immunocompromised due to HIV and other conditions. Primary immunodeficiency is not listed outright in the advisory, although “having autoimmune disease with immunodeficiency as a clinical component” is part of the advisory’s definition of other immunocompromising conditions.
The severe manifestations of monkeypox reported include extensive rashes with secondary bacterial or fungal skin infections or tissue death (necrosis), bowel blockage, and heart, lung, urinary, and neurological complications. Severe complications have occurred in people with healthy immune systems and those who are immunocompromised but are more likely in those who are immunocompromised. The advisory noted, “Of the people with severe manifestations of monkeypox for whom CDC has been consulted, the majority have had HIV with CD4 counts <200 cells/ml, indicating substantial immunosuppression.”
As of October 3, 2022, there have been 13 deaths in countries that have not historically reported monkeypox infections, including two deaths in the U.S. The number of cases is trending down globally and in the U.S., where there has been more than 25,000 total cases.
The advisory gives the following guidance for the public:
- If you are someone with a weakened immune system or are living with HIV and have been exposed to monkeypox or developed an illness or rash consistent with monkeypox, you should seek out testing and treatment for monkeypox from a healthcare provider.
- Tecovirimat (TPOXX) is currently recommended for people with severe monkeypox disease or who are at high risk of severe disease, like people with weakened immune systems, such as HIV that is not virally suppressed, or skin conditions, such as eczema.
Originally published August 4, 2022
U.S. Secretary of Health and Human Services Xavier Becerra has declared monkeypox a national public health emergency, just days after California, Illinois, and New York declared emergencies. The World Health Organization’s (WHO) Director-General declared the virus a Public Health Emergency of International Concern on July 23, 2022, though the WHO committee that typically makes that designation could not reach a consensus.
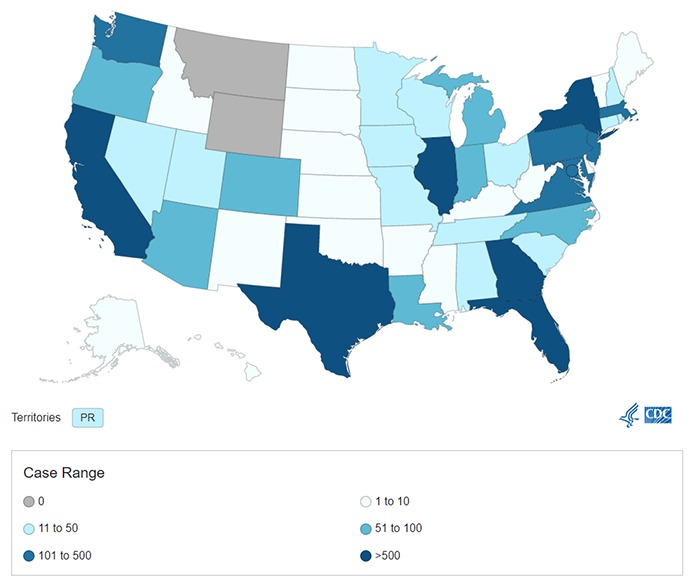
https://www.cdc.gov/poxvirus/monkeypox/response/2022/us-map.html
As of August 3, 2022, there are at least 25,864 confirmed cases of monkeypox in 80 countries where it has not been reported historically, including 6,617 cases in the U.S. This week has also seen the first deaths in this outbreak—two in Spain, and one each in Brazil and India.
Monkeypox is still circulating widely as global and U.S. public health officials struggle to get the current outbreak under control. The hope is that resources available under a public health emergency declaration, both internationally and in the U.S., will help turn the tide. Patients have reported delays in testing and diagnosis despite extensive media coverage, and vaccine rollout has been slow.
The U.S. Centers for Disease Control and Prevention (CDC) website lists the following “What You Need to Know” information:
- CDC is tracking an outbreak of monkeypox that has spread across several countries that don’t normally report monkeypox, including the United States.
- The monkeypox virus is spreading mostly through close, intimate contact with someone who has monkeypox.
- You can take steps to prevent getting monkeypox and lower your risk during sex.
- CDC recommends vaccination for people who have been exposed to monkeypox and people who are at higher risk of being exposed to monkeypox.
- Note that the ACAM2000 vaccine is contraindicated for people with immunocompromising conditions because it contains live virus that is able to replicate. The CDC recommends prioritizing JYNNEOS vaccine doses, which contain live virus that is not able to replicate, for this group.
- If you have any symptoms of monkeypox, talk to your healthcare provider, even if you don’t think you had contact with someone who has monkeypox.
- CDC is urging healthcare providers in the United States to be alert for patients who have rash illnesses consistent with monkeypox.
Originally published July 14, 2022
As of July 13, 2022, there are at least 10,845 confirmed cases of monkeypox in 59 countries where it has not been reported historically, including 1,053 cases in the U.S. On June 23, 2022, a committee of the World Health Organization (WHO) declined to declare the outbreak a Public Health Emergency of International Concern (PHEIC). However, the committee noted that individuals in the European region were at highest risk and recommended that the situation be reviewed again in several weeks.
In response to public concern over monkeypox and the safety of plasma-based therapeutics, the Plasma Protein Therapeutics Association (PPTA) has released a statement with the following conclusion: "Due to the characteristics of the virus and multiple, complementary steps with significant and robust virus removal and virus reduction capacity utilized during manufacturing of plasma protein therapies, PPTA considers that the current [monkeypox] outbreak is not a concern for the safety margins of plasma protein therapies manufactured by PPTA member companies."
Originally published May 31, 2022
Although these reports may bring early COVID-19 days to mind, there are key differences between the viruses that cause these diseases, and our familiarity with them, that make monkeypox unlikely to become widespread.
What is monkeypox?
NIAID, https://www.flickr.com/photos/niaid/52096775365.
Unlike COVID-19, monkeypox is not new to humans and scientists know quite a bit about the biology of the disease and how it spreads.
Monkeypox is an illness caused by a less dangerous cousin of the smallpox virus. It typically resolves on its own in immunocompetent people within 2-4 weeks. Patients first experience flu-like symptoms including fever, headache, muscle aches, and chills. Then a smallpox-like rash develops, usually starting on the face and spreading to other parts of the body. However, the World Health Organization notes that some patients in the current outbreak do not have typical symptoms. Sometimes, the rash is the first symptom, and there have been cases where the patient has only one or a handful of lesions.
A minority of patients develop serious disease and 1-10% die, depending on the viral strain. The first published viral sequence from the current outbreak indicates that the circulating strain is the less deadly of the two types of monkeypox virus. As with many viral illnesses, those who are immunocompromised are thought to be more vulnerable.
The monkeypox virus is zoonotic, which means it typically infects animals (in this case, rodents and primates) rather than people. However, in ‘endemic’ countries in West and Central Africa, the virus circulates between animals and humans and outbreaks have been reported since 1970. The virus can also spread from person to person through close contact with the rash, or large respiratory droplets. There is no evidence of airborne transmission, another key difference from COVID-19.
Finally, monkeypox is a DNA virus, rather than an RNA virus like SARS-CoV-2. Because of differences in how DNA viruses copy themselves, they are not as prone to giving rise to variants that could escape prior immunity.
Why is the current monkeypox outbreak newsworthy?
The current outbreak is unusual because it is occurring over a large geographical area in countries where monkeypox is extremely rare and typically found only in travelers. For example, the United Kingdom, which has 106 confirmed cases as of May 26, 2022, previously reported seven total monkeypox cases since 2018. Many of the current cases have no known travel to endemic countries and no known contact with people or animals from western or central Africa.
In a statement, the World Health Organization (WHO) said “The sudden appearance of monkeypox simultaneously in several non-endemic countries suggests that there may have been undetected transmission for some time as well as recent amplifying events.”
Another unusual factor in the current outbreak is that many of the confirmed cases are in men who have sex with men. Monkeypox is not considered a sexually-transmitted disease (STD) so this epidemiological pattern is unusual. However, it is possible that transmission is occurring because of close contact and/or exchange of respiratory droplets during sex and not, strictly speaking, because the virus is now being transmitted via semen or vaginal fluid.
What tools are effective against monkeypox?
The virus transmits through close contact and respiratory droplets and CDC Laboratory Response Network (LRN) sites in the U.S. and in other countries can test for active infection via detection of viral DNA. These factors make isolating monkeypox patients practical and one of the most effective ways to stop transmission.
Because monkeypox is closely related to smallpox, many of the vaccines and treatments originally developed for smallpox are also thought to be effective against monkeypox. For example, people previously vaccinated against smallpox (which was part of routine vaccination in the U.S. until 1972) have some cross-protection against monkeypox.
In addition, vaccines and treatments for smallpox are available through government stockpiles. The U.S. currently licenses two smallpox vaccines, ACAM2000 and JYNNEOS (JYNNEOS is also licensed to treat monkeypox). On May 25, 2022, the U.S. government released vaccines for close contacts of confirmed cases in a strategy called ring vaccination that is designed to stop the spread of the virus. Unfortunately, both vaccines contain live virus and may be contraindicated for many with primary immunodeficiency.
There are also antiviral medications that are likely effective against monkeypox. Brincidofovir was approved by the U.S. Food and Drug Associaton for the treatment of smallpox, and has been shown to be effective in animal studies. In a retrospective case study, one British patient treated with tecovirimat, another smallpox antiviral that prevents infected cells from producing new viral particles, had a shorter duration of illness and viral shedding.
What’s the bottom line?
It should be noted that even in endemic countries, monkeypox is a rare disease. The Democratic Republic of the Congo (DRC) reported 6,257 suspected cases in 2020, compared to an estimated 29 million cases of malaria. As Dr. Mark Ballow, IDF’s medical advisor, puts it, “Risk to the general public is low, but you should seek medical care immediately if you develop a new, unexplained skin rash (lesions on any part of the body), with or without fever and chills, and avoid contact with others.”
Ballow also suggests calling ahead before visiting a medical facility if you suspect you have monkeypox. If applicable, be sure to include the following information in your discussion with your healthcare provider:
- Any contact with a person that might have had monkeypox.
- If you are a man who has had intimate contact (including sex) with other men.
- Travel to an area where monkeypox has been reported (see countries listed by WHO) or in an area where monkeypox is more commonly found (the Democratic Republic of the Congo, Republic of the Congo, Nigeria, Central African Republic, Cameroon, Côte d’Ivoire, Gabon, Liberia, Sierra Leone, or Sudan).
Protective measures you can take include avoiding crowded places, social distancing when possible, and using a mask — all measures that are also effective against COVID-19, which is still actively circulating in most places.
Related resources
Sign up for updates from IDF
Receive news and helpful resources to your cell phone or inbox. You can change or cancel your subscription at any time.





The Immune Deficiency Foundation improves the diagnosis, treatment, and quality of life for every person affected by primary immunodeficiency.
We foster a community that is connected, engaged, and empowered through advocacy, education, and research.
Combined Charity Campaign | CFC# 66309




