
-
Understanding primary immunodeficiency (PI)

Understanding PI
The more you understand about primary immunodeficiency (PI), the better you can manage it. Learn about PI diagnoses and treatment options.
-
Living with PI
-
Addressing mental health
-
Explaining your diagnosis
- General care
- Get support
- For parents and guardians
-
Managing workplace issues
- Navigating insurance
-
Traveling safely

Living with PI
Living with primary immunodeficiency (PI) can be challenging, but you’re not alone—many people with PI lead full and active lives. With the right support and resources, you can, too.
-
Addressing mental health
-
Get involved

Get involved
Be a hero for those with PI. Change lives by promoting primary immunodeficiency (PI) awareness and taking action in your community through advocacy, donating, volunteering, or fundraising.
-
Advancing research and clinical care
-
Research Grant Program
-
Consulting immunologist
-
Diagnosing PI
-
Getting prior authorization
-
Clinician education
-
Survey research
-
Participating in clinical trials

Advancing research and clinical care
Whether you’re a clinician, researcher, or an individual with primary immunodeficiency (PI), IDF has resources to help you advance the field. Get details on surveys, grants, and clinical trials.
-
Research Grant Program
This article, originally published on July 21, 2023, has been updated:
- Aug. 14, 2024: To include Moderna's new RSV vaccine, mRESVIA, to note that Arexvy is approved for those ages 50-59 at high risk of lower respiratory tract disease from RSV, and to reflect changes to vaccine recommendations.
- Oct. 23, 2024: To note that Abrysvo is approved for those ages 18-59 at high risk of lower respiratory tract disease from RSV.
- June 10, 2025: To include Merck's monoclonal antibody Enflonsia for use in infants.
- June 12, 2025: To note that mResvia is approved for those ages 18-59 at high risk of lower respiratory tract disease from RSV.
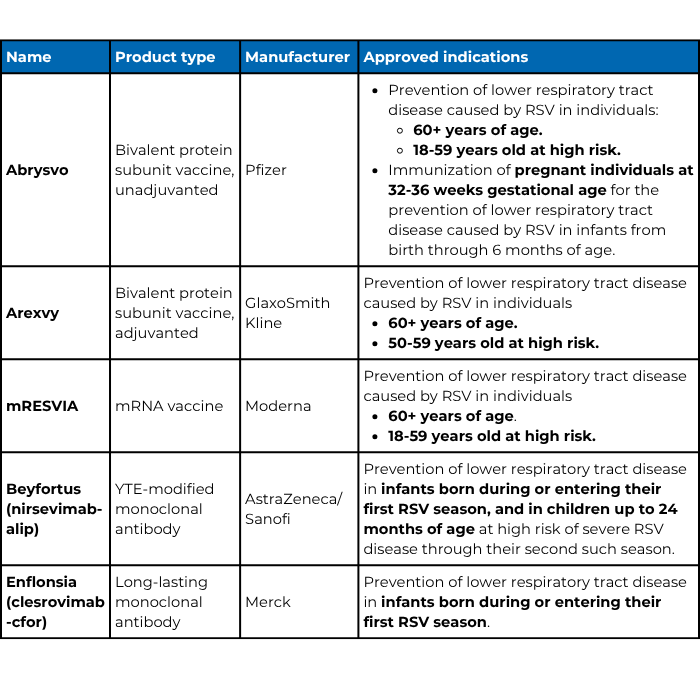
Respiratory syncytial virus (RSV) is the most common cause of lower respiratory tract infections in babies and young children. It also causes infections in older adults and adults with chronic conditions. Until recently, respiratory virus hygiene, including handwashing, was the most effective RSV prevention for the general public. But, the U.S. Food and Drug Administration (FDA) has approved five preventative products for RSV over the last two years:
- Abrysvo and Arexvy, two protein subunit vaccines.
- mRESVIA, an mRNA vaccine.
- Beyfortus and Enflonsia, long-lasting monoclonal antibodies.
RSV and primary immunodeficiency
In the U.S., RSV season typically lasts from October through April, with a peak in December or January. Symptoms include runny nose, coughing, sneezing, fever, and difficulty breathing. RSV has two subtypes, A and B, that differ genetically, but there is no difference in disease severity between them.
The U.S. Centers for Disease Control and Prevention (CDC) estimates that between 58,000-80,000 children under age 5 and 100,000-150,000 adults 65 and over are hospitalized with RSV each year, mainly for breathing difficulty and dehydration. While most people recover, in the 2024-2025 season, an estimated 10,000-23,000 people ultimately die from the infection or its complications. Along with chronic heart and lung disease, being immunocompromised is a risk factor for developing severe RSV.
Research on RSV in those with primary immunodeficiency (PI) has primarily focused on infants and children. A 2012 paper found that children with PI are 3.8 times more likely to be hospitalized for an RSV infection than children without a chronic condition. Studies in Japan and Spain found that, in particular, children with PIs that affect T cells (for example, combined immunodeficiencies) are at the greatest risk of hospitalization for RSV.
Preventing RSV infections
Prior to May 2023, the only FDA-approved product for the prevention of lower respiratory tract infections from RSV was palivizumab, a monoclonal antibody manufactured by Swedish Orphan Biovitrum under the trade name Synagis. Monoclonal antibodies provide passive immunity and work by binding to and blocking germs so they can’t infect cells. However, palivizumab is only approved for certain high-risk infants and is typically given as a monthly injection for up to five consecutive months during RSV season.
Technological breakthroughs have led to an explosion of clinical research on new prevention and treatment products for RSV over the last decade. As of June 2025, there are three approved RSV vaccines for adults and two long-lasting monoclonal antibodies for infants.
Options for adults
Abrysvo, Arexvy, and mRESVIA are all vaccines, which means they rely on a person’s immune system to generate antibodies and/or T cells that will protect that person from RSV infection. All three are approved for people who are 60 years old and older. Arexvy is also approved for people 50-59 years of age who are at high risk, including those who are immunocompromised. As of October 2024, the U.S. Food and Drug Administration (FDA) expanded Abrysvo's approval to those 18-59 years of age who are at high risk of lower respiratory tract infection from RSV as well. mRESVIA was approved for those ages 18-59 at high risk in June 2025.
Importantly, none of the three vaccines contain live virus (or even whole virus), so they cannot cause RSV infections in individuals with PI. Likewise, household members and other close contacts who have been vaccinated cannot spread RSV to individuals with PI.
There are key differences between the three vaccines. Abrysvo and Arexvy are protein subunit vaccines that contain a piece of RSV meant to provoke an immune response—in this case, a protein found on the virus surface that helps RSV infect human cells. Arexvy also contains an adjuvant, which is a substance added to vaccines that boosts a person’s immune response, whereas Abrysvo does not.
In phase III clinical trials, Arexvy was 82.6% effective at preventing lower respiratory tract infections from RSV in adults aged 60 and older during their first RSV season after vaccination. Recently, GlaxoSmithKline published additional analysis of Arexvy in those 60 and older with at least one chronic condition placing them at high risk of severe RSV. These conditions included any chronic respiratory or pulmonary disease (including COPD and asthma), chronic heart failure, diabetes mellitus type 1 or type 2, and advanced liver or renal disease. Among this population, Arexvy was 94.6% effective in preventing lower respiratory tract disease from RSV. Unpublished data submitted to the FDA to expand approval of Arexvy in younger individuals found that those 50-59 years old with certain chronic conditions produced similar antibody responses as the older individuals in the phase III trial.
Abrysvo was 66.7% effective in a phase III clinical trial in adults aged 60 and older during their first RSV season after vaccination. In those ages 18-59 at high risk, Abrysvo produced a similar antibody response as the older individuals in the phase III trial, leading the FDA to approve Abrysvo for this population as well.
In contrast to the other two vaccines, mRESVIA is an mRNA vaccine similar to the mRNA COVID-19 vaccines. It contains instructions (mRNA) that code for the same RSV surface protein Abrysvo and Arexvy contain. mRESVIA relies on your cells to use the mRNA to make the surface protein, which then provokes an immune response. mRESVIA was 83.7% effective in preventing lower respiratory tract infections from RSV in adults 60 years of age or older during their first RSV season after vaccination.
Note that because each trial was done independently with different criteria, the efficacy of the vaccines cannot be compared directly.
Based on these trials, the CDC’s Advisory Committee on Immunization Practices (ACIP) recommends that everyone 75 and older receive an RSV vaccine in late summer or early fall before RSV season begins. ACIP also recommends that those 60-74 years old who are at high risk from RSV get an RSV vaccine.
At this time, whether individuals with PI produce a protective response to the RSV vaccines is unknown. Experience with COVID-19 vaccines suggests that vaccination against RSV may protect at least some individuals with PI. IDF’s Medical Advisory Committee agrees with ACIP’s recommendations and encourages those with PI to speak with their healthcare provider about the vaccines, particularly if they have chronic lung or heart disease.
Options for infants
Young children are also at risk for severe RSV. Two passive forms of immunity, which do not rely on an individual’s immune response, are now available to protect infants: maternal vaccination with Abrysvo or direct infusion of long-lasting monoclonal antibodies directly to infants. The monoclonal antibodies available include nirsevimab, made by AstraZeneca and Sanofi under the trade name Beyfortus, and clesrovimab, made by Merck under the trade name Enflonsia.
For maternal vaccination, pregnant individuals are vaccinated so that they pass protective antibodies through the placenta to their babies. In the Abrysvo maternal vaccination trial, vaccination at 24-36 weeks of pregnancy was 81.8% effective in preventing severe RSV in newborns from birth through 6 months of age. Note, however, that pregnant individuals with a PI may not develop protective antibodies from Abrysvo vaccination to pass on.
Nirsevimab is a monoclonal antibody that targets the same RSV surface protein as palivizumab and all three vaccines, blocking RSV’s ability to infect human cells. In phase IIb and phase III clinical trials in premature and term infants, a single shot of nirsevimab was 70.1-74.9% effective over five months in preventing RSV lower respiratory tract infections that required medical attention.
Interestingly, nirsevimab uses the same YTE technology as AstraZeneca’s Evusheld to stabilize the antibody and prevent it from breaking down in the bloodstream. This modification allows it to be given as a single intramuscular shot that lasts for the duration of a single RSV season.
Clesrovimab is also a long-lasting monoclonal antibody that uses the same stabilizing YTE technology. In phase IIb and phase III clinical trials in premature and term infants, a single shot of clesrovimab was 60.5% effective over five months in preventing RSV lower respiratory tract infections that required medical attention.
To protect infants born during RSV season, the CDC recommends either maternal vaccination at 32-36 weeks gestation or immunization with nirsevimab within the first week after birth. Babies born between RSV seasons whose mothers were not vaccinated should receive nirsevimab if they are 8 months old or younger at the start of the next RSV season. In addition, high-risk babies up to 24 months old, including those who are severely immunocompromised, entering their second RSV season should also receive nirsevimab. Talk to your doctor to see if your child should receive the antibody prior to their second RSV season. Although the FDA has approved clesrovimab, it has not yet been incorporated into the CDC's recommendation.
Browse articles
The Immune Deficiency Foundation publishes articles on a wide variety of topics, including policy, community profiles, and the latest research on PI. Find your next read!
Browse articlesThis page contains general medical and/or legal information that cannot be applied safely to any individual case. Medical and/or legal knowledge and practice can change rapidly. Therefore, this page should not be used as a substitute for professional medical and/or legal advice. Additionally, links to other resources and websites are shared for informational purposes only and should not be considered an endorsement by the Immune Deficiency Foundation.
Related resources

Foundation provides guidance amid changing vaccine recommendations

Education policy changes could impact students with PI
Foundation to follow medical societies' vaccine schedules in light of ACIP hepatitis B decision
Sign up for updates from IDF
Receive news and helpful resources to your cell phone or inbox. You can change or cancel your subscription at any time.




The Immune Deficiency Foundation improves the diagnosis, treatment, and quality of life for every person affected by primary immunodeficiency.
We foster a community that is connected, engaged, and empowered through advocacy, education, and research.

